FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development
- PMID: 21084449
- PMCID: PMC3219046
- DOI: 10.1210/en.2010-0636
FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development
Abstract
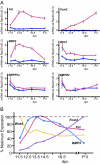
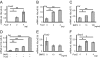
Follistatin is a secreted glycoprotein required for female sex determination and early ovarian development, but the precise mechanisms regulating follistatin (Fst) gene expression are not known. Here, we investigate the roles of bone morphogenetic protein 2 (BMP2) and forkhead-domain transcription factor L2 (FOXL2) in the regulation of Fst expression in the developing mouse ovary. Bmp2 and Fst showed similar temporal profiles of mRNA expression, whereas FOXL2 protein and Fst mRNA were coexpressed in the same ovarian cells. In a cell culture model, both FOXL2 and BMP2 up-regulated Fst expression. In ex vivo mouse fetal gonad culture, exogenous BMP2 increased Fst expression, but this effect was counteracted by the BMP antagonist Noggin. Moreover, in Foxl2-null mice, Fst expression was reduced throughout fetal ovarian development, and Bmp2 expression was also reduced. Our data support a model in which FOXL2 and BMP2 cooperate to ensure correct expression of Fst in the developing ovary. Further, Wnt4-knockout mice showed reduced expression of Fst limited to early ovarian development, suggesting a role for WNT4 in the initiation, but not the maintenance, of Fst expression.
Figures

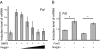
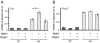

References
-
- Wilhelm D, Palmer S, Koopman P 2007 Sex determination and gonadal development in mammals. Physiol Rev 87:1–28 - PubMed
-
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R 1991 Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121 - PubMed
-
- Menke DB, Page DC 2002 Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns 2:359–367 - PubMed
-
- Phillips DJ, de Kretser DM 1998 Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol 9:287–322 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

