Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera
- PMID: 21084458
- PMCID: PMC3019776
- DOI: 10.1128/CVI.00370-10
Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera
Abstract
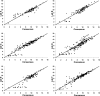
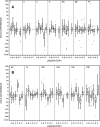
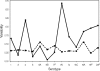
Antibody-mediated killing of Streptococcus pneumoniae (pneumococcus) by phagocytes is an important mechanism of protection of the human host against pneumococcal infections. Measurement of opsonophagocytic antibodies by use of a standardized opsonophagocytic assay (OPA) is important for the evaluation of candidate vaccines and required for the licensure of new pneumococcal conjugate vaccine formulations. We assessed agreement among six laboratories that used their own optimized OPAs on a panel of 16 human reference sera for 13 pneumococcal serotypes. Consensus titers, estimated using an analysis-of-variance (ANOVA) mixed-effects model, provided a common reference for assessing agreement among these laboratories. Agreement was evaluated in terms of assay accuracy, reproducibility, repeatability, precision, and bias. We also reviewed four acceptance criterion intervals for assessing the comparability of protocols when assaying the same reference sera. The precision, accuracy, and concordance results among laboratories and the consensus titers revealed acceptable agreement. The results of this study indicate that the bioassays evaluated in this study are robust, and the resultant OPA values are reproducible for the determination of functional antibody titers specific to 13 pneumococcal serotypes when performed by laboratories using highly standardized but not identical assays. The statistical methodologies employed in this study may serve as a template for evaluating future multilaboratory studies.
Figures

Similar articles
-
Interlaboratory Comparison of the Pneumococcal Multiplex Opsonophagocytic Assays and Their Level of Agreement for Determination of Antibody Function in Pediatric Sera.mSphere. 2018 Apr 25;3(2):e00070-18. doi: 10.1128/mSphere.00070-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29695620 Free PMC article.
-
Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae.Clin Diagn Lab Immunol. 2005 Feb;12(2):287-95. doi: 10.1128/CDLI.12.2.287-295.2005. Clin Diagn Lab Immunol. 2005. PMID: 15699424 Free PMC article.
-
Assignment of Opsonic Values to Pneumococcal Reference Serum 007sp for Use in Opsonophagocytic Assays for 13 Serotypes.Clin Vaccine Immunol. 2017 Feb 6;24(2):e00457-16. doi: 10.1128/CVI.00457-16. Print 2017 Feb. Clin Vaccine Immunol. 2017. PMID: 27974397 Free PMC article.
-
Creation, characterization, and assignment of opsonic values for a new pneumococcal OPA calibration serum panel (Ewha QC sera panel A) for 13 serotypes.Medicine (Baltimore). 2018 Apr;97(17):e0567. doi: 10.1097/MD.0000000000010567. Medicine (Baltimore). 2018. PMID: 29703046 Free PMC article.
-
Pneumococcal vaccine and opsonic pneumococcal antibody.J Infect Chemother. 2013 Jun;19(3):412-25. doi: 10.1007/s10156-013-0601-1. Epub 2013 May 9. J Infect Chemother. 2013. PMID: 23657429 Free PMC article. Review.
Cited by
-
Interlaboratory Comparison of the Pneumococcal Multiplex Opsonophagocytic Assays and Their Level of Agreement for Determination of Antibody Function in Pediatric Sera.mSphere. 2018 Apr 25;3(2):e00070-18. doi: 10.1128/mSphere.00070-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29695620 Free PMC article.
-
A Nonadjuvanted Whole-Inactivated Pneumococcal Vaccine Induces Multiserotype Opsonophagocytic Responses Mediated by Noncapsule-Specific Antibodies.mBio. 2022 Oct 26;13(5):e0236722. doi: 10.1128/mbio.02367-22. Epub 2022 Sep 20. mBio. 2022. PMID: 36125268 Free PMC article.
-
Serotype-independent pneumococcal vaccines.Cell Mol Life Sci. 2013 Sep;70(18):3303-26. doi: 10.1007/s00018-012-1234-8. Epub 2012 Dec 27. Cell Mol Life Sci. 2013. PMID: 23269437 Free PMC article. Review.
-
An Experimental Group A Streptococcus Vaccine That Reduces Pharyngitis and Tonsillitis in a Nonhuman Primate Model.mBio. 2019 Apr 30;10(2):e00693-19. doi: 10.1128/mBio.00693-19. mBio. 2019. PMID: 31040243 Free PMC article.
-
The development of functional opsonophagocytic assays to evaluate antibody responses to Klebsiella pneumoniae capsular antigens.mSphere. 2025 Jul 29;10(7):e0017625. doi: 10.1128/msphere.00176-25. Epub 2025 Jun 12. mSphere. 2025. PMID: 40503906 Free PMC article.
References
-
- Bogaert, D., M. Sluijter, R. de Groot, and P. W. M. Hermans. 2004. Multiplex opsonophagocytosis assay (MOPA): a useful tool for the monitoring of the 7-valent pneumococcal conjugate vaccine. Vaccine 4563:1-7. - PubMed
-
- Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 46(RR-8):1-24. - PubMed
-
- Centers for Disease Control and Prevention. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 49(RR-09):1-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

