Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity
- PMID: 21084461
- PMCID: PMC3019785
- DOI: 10.1128/CVI.00341-10
Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity
Abstract
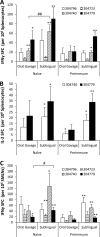
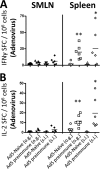
HIV/AIDS continue to devastate populations worldwide. Recent studies suggest that vaccines that induce beneficial immune responses in the mucosal compartment may improve the efficacy of HIV vaccines. Adenovirus serotype 5 (Ad5)-based vectors remain a promising platform for the development of effective vaccines. In an effort to improve the efficacy of Ad5-based vaccines, even in the presence of preexisting Ad5 immunity, we evaluated the potential for an Ad5-based HIV vaccine to induce antigen-specific immune responses following sublingual (s.l.) administration, a route not previously tested in regard to Ad-based vaccines. s.l. vaccination with an Ad5-based HIV-Gag vaccine resulted in a significant induction of Gag-specific cytotoxic T-lymphocyte (CTL) responses in both the systemic and the mucosal compartment. We also show that s.l. immunization not only avoided preexisting Ad5 immunity but also elicited a broad repertoire of antigen-specific CTL clones. Additionally, we confirm for the first time that oral delivery of a vaccine expressing a potent Toll-like receptor (TLR) agonist can stimulate innate immune responses through induction of cytokines and chemokines and activation of NK cells, NKT cells, and macrophages in vivo. These results positively correlated with improved antigen-specific CTL responses. These results could be achieved both in Ad5-naïve mice and in mice with preexisting immunity to Ad5. The simplicity of the s.l. vaccination regimen coupled with augmentation of TLR-dependent pathways active in the oral cavity makes s.l. delivery a promising method for HIV vaccine development specifically, as well as for many other vaccine applications in general.
Figures



Similar articles
-
A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target.PLoS One. 2010 Mar 8;5(3):e9579. doi: 10.1371/journal.pone.0009579. PLoS One. 2010. PMID: 20221448 Free PMC article.
-
Natural killer T cell and TLR9 agonists as mucosal adjuvants for sublingual vaccination with clade C HIV-1 envelope protein.Vaccine. 2014 Dec 5;32(51):6934-6940. doi: 10.1016/j.vaccine.2014.10.051. Epub 2014 Nov 2. Vaccine. 2014. PMID: 25444819 Free PMC article.
-
Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene.J Virol. 2003 Jun;77(11):6305-13. doi: 10.1128/jvi.77.11.6305-6313.2003. J Virol. 2003. PMID: 12743287 Free PMC article.
-
Induction of intestinal immunity by mucosal vaccines as a means of controlling HIV infection.AIDS Res Hum Retroviruses. 2014 Nov;30(11):1027-40. doi: 10.1089/aid.2014.0233. AIDS Res Hum Retroviruses. 2014. PMID: 25354023 Free PMC article. Review.
-
The influence of delivery vectors on HIV vaccine efficacy.Front Microbiol. 2014 Aug 22;5:439. doi: 10.3389/fmicb.2014.00439. eCollection 2014. Front Microbiol. 2014. PMID: 25202303 Free PMC article. Review.
Cited by
-
Sublingual Immunization with Chimeric C1q/CD40 Ligand/HIV Virus-like Particles Induces Strong Mucosal Immune Responses against HIV.Vaccines (Basel). 2021 Oct 23;9(11):1236. doi: 10.3390/vaccines9111236. Vaccines (Basel). 2021. PMID: 34835167 Free PMC article.
-
Microneedle-Mediated Vaccine Delivery to the Oral Mucosa.Adv Healthc Mater. 2019 Feb;8(4):e1801180. doi: 10.1002/adhm.201801180. Epub 2018 Dec 10. Adv Healthc Mater. 2019. PMID: 30537400 Free PMC article. Review.
-
Challenges in mucosal HIV vaccine development: lessons from non-human primate models.Viruses. 2014 Aug 15;6(8):3129-58. doi: 10.3390/v6083129. Viruses. 2014. PMID: 25196380 Free PMC article. Review.
-
Buccal and sublingual vaccine delivery.J Control Release. 2014 Sep 28;190:580-92. doi: 10.1016/j.jconrel.2014.05.060. Epub 2014 Jun 6. J Control Release. 2014. PMID: 24911355 Free PMC article. Review.
-
Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually.Vaccine. 2015 Oct 26;33(43):5845-5853. doi: 10.1016/j.vaccine.2015.08.086. Epub 2015 Sep 21. Vaccine. 2015. PMID: 26392012 Free PMC article.
References
-
- Ahlers, J. D., and I. M. Belyakov. 2010. Lessons learned from natural infection: focusing on the design of protective T cell vaccines for HIV/AIDS. Trends Immunol. 31:120-130. - PubMed
-
- Appledorn, D. M., et al. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134-2144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

