Early events in retrovirus XMRV infection of the wild-derived mouse Mus pahari
- PMID: 21084477
- PMCID: PMC3020513
- DOI: 10.1128/JVI.00886-10
Early events in retrovirus XMRV infection of the wild-derived mouse Mus pahari
Abstract
A novel gammaretrovirus, xenotropic murine leukemia virus-related virus (XMRV), has been identified in patients with prostate cancer and in patients with chronic fatigue syndromes. Standard Mus musculus laboratory mice lack a functional XPR1 receptor for XMRV and are therefore not a suitable model for the virus. In contrast, Gairdner's shrew-mice (Mus pahari) do express functional XPR1. To determine whether Mus pahari could serve as a model for XMRV, primary Mus pahari fibroblasts and mice were infected with cell-free XMRV. Infection of cells in vitro resulted in XMRV Gag expression and the production of XMRV virions. After intraperitoneal injection of XMRV into Mus pahari mice, XMRV proviral DNA could be detected in spleen, blood, and brain. Intravenous administration of a green fluorescent protein (GFP) vector pseudotyped with XMRV produced GFP(+) CD4(+) T cells and CD19(+) B cells. Mice mounted adaptive immune responses against XMRV, as evidenced by the production of neutralizing and Env- and Gag-specific antibodies. Prominent G-to-A hypermutations were also found in viral genomes isolated from the spleen, suggesting intracellular restriction of XMRV infection by APOBEC3 in vivo. These data demonstrate infection of Mus pahari by XMRV, potential cell tropism of the virus, and immunological and intracellular restriction of virus infection in vivo. These data support the use of Mus pahari as a model for XMRV pathogenesis and as a platform for vaccine and drug development against this potential human pathogen.
Figures
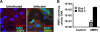


References
-
- Caligiuri, M., et al. 1987. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J. Immunol. 139:3306-3313. - PubMed
-
- Cloyd, M. W., M. M. Thompson, and J. W. Hartley. 1985. Host range of mink cell focus-inducing viruses. Virology 140:239-248. - PubMed
-
- D'Arcy, F., et al. 2008. No evidence of XMRV in Irish prostate cancer patients with the R462Q mutation. Eur. Urol. Suppl. 7:271.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

