The nuclear and adherent junction complex component protein ubinuclein negatively regulates the productive cycle of Epstein-Barr virus in epithelial cells
- PMID: 21084479
- PMCID: PMC3020007
- DOI: 10.1128/JVI.01397-10
The nuclear and adherent junction complex component protein ubinuclein negatively regulates the productive cycle of Epstein-Barr virus in epithelial cells
Abstract
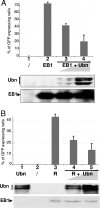
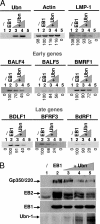
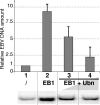
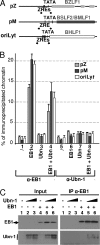
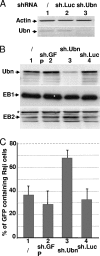
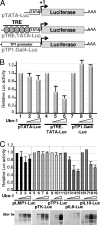
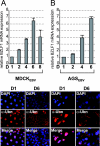
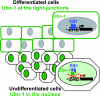
The Epstein-Barr Virus (EBV) productive cycle is initiated by the expression of the viral trans-activator EB1 (also called Zebra, Zta, or BZLF1), which belongs to the basic leucine zipper transcription factor family. We have previously identified the cellular NACos (nuclear and adherent junction complex components) protein ubinuclein (Ubn-1) as a partner for EB1, but the function of this complex has never been studied. Here, we have evaluated the consequences of this interaction on the EBV productive cycle and find that Ubn-1 overexpression represses the EBV productive cycle whereas Ubn-1 downregulation by short hairpin RNA (shRNA) increases virus production. By a chromatin immunoprecipitation (ChIP) assay, we show that Ubn-1 blocks EB1-DNA interaction. We also show that in epithelial cells, relocalization and sequestration of Ubn-1 to the tight junctions of nondividing cells allow increased activation of the productive cycle. We propose a model in which Ubn-1 is a modulator of the EBV productive cycle: in proliferating epithelial cells, Ubn-1 is nuclear and inhibits activation of the productive cycle, whereas in differentiated cells, Ubn-1 is sequestrated to tight junctions, thereby allowing EB1 to fully function in the nucleus.
Figures
References
-
- Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. - PMC - PubMed
-
- Aho, S., J. Lupo, P. A. Coly, A. Sabine, M. Castellazzi, P. Morand, A. Sergeant, E. Manet, V. Boyer, and H. Gruffat. 2009. Characterization of the ubinuclein protein as a new member of the nuclear and adhesion complex components (NACos). Biol. Cell 101:319-334. - PubMed
-
- Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

