Viral exploitation of host SOCS protein functions
- PMID: 21084484
- PMCID: PMC3067810
- DOI: 10.1128/JVI.01857-10
Viral exploitation of host SOCS protein functions
Abstract
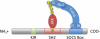
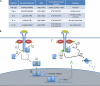
Over the past decade, a family of host proteins known as suppressors of cytokine signaling (SOCS) have emerged as frequent targets of viral exploitation. Under physiologic circumstances, SOCS proteins negatively regulate inflammatory signaling pathways by facilitating ubiquitination and proteosomal degradation of pathway machinery. Their expression is tightly regulated to prevent excessive inflammation while maintaining protective antipathogenic responses. Numerous viruses, however, have developed mechanisms to induce robust host SOCS protein expression following infection, essentially "hijacking" SOCS function to promote virus survival. To date, SOCS proteins have been shown to inhibit protective antiviral signaling pathways, allowing viruses to evade the host immune response, and to ubiquitinate viral proteins, facilitating intracellular viral trafficking and progeny virus assembly. Importantly, manipulation of SOCS proteins not only facilitates progression of the viral life cycle but also powerfully shapes the presentation of viral disease. SOCS proteins can define host susceptibility to infection, contribute to peripheral disease manifestations such as immune dysfunction and cancer, and even modify the efficacy of therapeutic interventions. Looking toward the future, it is clear that a better understanding of the role of SOCS proteins in viral diseases will be essential in our struggle to modulate and even eliminate the pathogenic effects of viruses on the host.
Figures
References
-
- Bansal, G. P., A. Malaspina, and J. Flores. 2010. Future paths for HIV vaccine research: exploiting results from recent clinical trials and current scientific advances. Curr. Opin. Mol. Ther. 12:39-46. - PubMed
-
- Biggs, B. A., M. Hewish, S. Kent, K. Hayes, and S. M. Crowe. 1995. HIV-1 infection of human macrophages impairs phagocytosis and killing of Toxoplasma gondii. J. Immunol. 154:6132-6139. - PubMed
-
- Bode, J. G., E. D. Brenndorfer, and D. Haussinger. 2007. Subversion of innate host antiviral strategies by the hepatitis C virus. Arch. Biochem. Biophys. 462:254-265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

