Three Drosophila liprins interact to control synapse formation
- PMID: 21084592
- PMCID: PMC2999520
- DOI: 10.1523/JNEUROSCI.1862-10.2010
Three Drosophila liprins interact to control synapse formation
Abstract
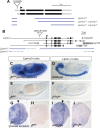
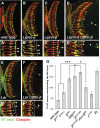
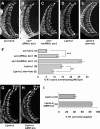
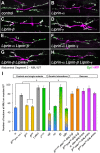
Liprin-α proteins are adaptors that interact with the receptor protein tyrosine phosphatase leukocyte common antigen-related (LAR) and other synaptic proteins to promote synaptic partner selection and active zone assembly. Liprin-β proteins bind to and share homology with Liprin-α proteins, but their functions at the synapse are unknown. The Drosophila genome encodes single Liprin-α and Liprin-β homologs, as well as a third related protein that we named Liprin-γ. We show that both Liprin-β and Liprin-γ physically interact with Liprin-α and that Liprin-γ also binds to LAR. Liprin-α mutations have been shown to disrupt synaptic target layer selection by R7 photoreceptors and to reduce the size of larval neuromuscular synapses. We have generated null mutations in Liprin-β and Liprin-γ to investigate their role in these processes. We find that, although Liprin-α mutant R7 axons terminate before reaching the correct target layer, Liprin-β mutant R7 axons grow beyond their target layer. Larval neuromuscular junction size is reduced in both Liprin-α and Liprin-β mutants, and further reduced in double mutants, suggesting independent functions for these Liprins. Genetic interactions demonstrate that both Liprin proteins act through the exchange factor Trio to promote stable target selection by R7 photoreceptor axons and growth of neuromuscular synapses. Photoreceptor and neuromuscular synapses develop normally in Liprin-γ mutants; however, removing Liprin-γ improves R7 targeting in Liprin-α mutants, and restores normal neuromuscular junction size to Liprin-β mutants, suggesting that Liprin-γ counteracts the functions of the other two Liprins. We propose that context-dependent interactions between the three Liprins modulate their functions in synapse formation.
Figures




References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating Trio expression in motor neurons. Neuron. 2010;66:536–549. - PubMed
-
- Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
