Motor and dorsal root ganglion axons serve as choice points for the ipsilateral turning of dI3 axons
- PMID: 21084609
- PMCID: PMC6633670
- DOI: 10.1523/JNEUROSCI.2380-10.2010
Motor and dorsal root ganglion axons serve as choice points for the ipsilateral turning of dI3 axons
Abstract
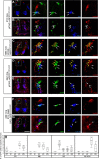
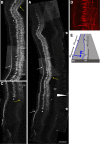
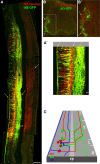
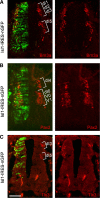
The axons of the spinal intersegmental interneurons are projected longitudinally along various funiculi arrayed along the dorsal-ventral axis of the spinal cord. The roof plate and the floor plate have a profound role in patterning their initial axonal trajectory. However, other positional cues may guide the final architecture of interneuron tracks in the spinal cord. To gain more insight into the organization of specific axonal tracks in the spinal cord, we focused on the trajectory pattern of a genetically defined neuronal population, dI3 neurons, in the chick spinal cord. Exploitation of newly characterized enhancer elements allowed specific labeling of dI3 neurons and axons. dI3 axons are projected ipsilaterally along two longitudinal fascicules at the ventral lateral funiculus (VLF) and the dorsal funiculus (DF). dI3 axons change their trajectory plane from the transverse to the longitudinal axis at two novel checkpoints. The axons that elongate at the DF turn at the dorsal root entry zone, along the axons of the dorsal root ganglion (DRG) neurons, and the axons that elongate at the VLF turn along the axons of motor neurons. Loss and gain of function of the Lim-HD protein Isl1 demonstrate that Isl1 is not required for dI3 cell fate. However, Isl1 is sufficient to impose ipsilateral turning along the motor axons when expressed ectopically in the commissural dI1 neurons. The axonal patterning of dI3 neurons, revealed in this study, highlights the role of established axonal cues-the DRG and motor axons-as intermediate guidepost cues for dI3 axons.
Figures




References
-
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. - PubMed
-
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardó M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
