Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences
- PMID: 21084614
- PMCID: PMC3004096
- DOI: 10.1523/JNEUROSCI.2871-10.2010
Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences
Abstract
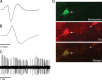
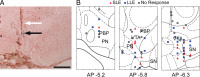
Recent studies show that glutamate and orexin (ORX, also known as hypocretin) inputs to the ventral tegmental area (VTA) dopamine (DA) cell region are essential for conditioned behavioral responses to reward-associated stimuli. In vitro experiments showed that ORX inputs to VTA potentiate responses of DA neurons to glutamate inputs, but it has remained unclear which glutamate inputs are modulated by ORX. The medial prefrontal cortex (mPFC) is a good candidate, given its role in processing complex stimulus-response information and its reciprocal connections with VTA DA neurons. Here we used in vivo recordings in anesthetized rats to investigate the responses of VTA DA neurons to mPFC stimulation, and how these responses are modulated by ORX. We demonstrate that mPFC stimulation evokes short- and long-latency excitation and inhibition in DA neurons. Maximal short-latency excitatory responses originated from stimulation sites in ventral prelimbic/infralimbic cortex, and were significantly more frequent during the active than during the rest period of the diurnal cycle. Application of ORX onto VTA DA neurons increased baseline activity and augmented or revealed excitatory responses to mPFC stimulation independent of changes in baseline activity, and without consistently affecting inhibitory responses. Moreover, orexin-1 receptor antagonism decreased tonic DA cell activity in active- but not rest-period animals, confirming a diurnal influence of ORX. These results indicate that ORX potently influences DA neuron activity, in part by modulating responses to mPFC inputs. By regulating prefrontal control of DA release, ORX projections to VTA may shape motivated behaviors in response to conditioned stimuli.
Figures









References
-
- Almodóvar-Fabregas LJ, Segarra O, Colón N, Dones JG, Mercado M, Mejías-Aponte CA, Vázquez R, Abreu R, Vázquez E, Williams JT, Jiménez-Rivera CA. Effects of cocaine administration on VTA cell activity in response to prefrontal cortex stimulation. Ann N Y Acad Sci. 2002;965:157–171. - PubMed
-
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
