Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion
- PMID: 21084621
- PMCID: PMC3001271
- DOI: 10.1523/JNEUROSCI.4340-10.2010
Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion
Abstract
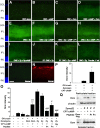
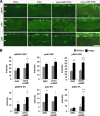
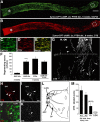
The inability of retinal ganglion cells (RGCs) to regenerate damaged axons through the optic nerve has dire consequences for victims of traumatic nerve injury and certain neurodegenerative diseases. Several strategies have been shown to induce appreciable regeneration in vivo, but the regrowth of axons through the entire optic nerve and on into the brain remains a major challenge. We show here that the induction of a controlled inflammatory response in the eye, when combined with elevation of intracellular cAMP and deletion of the gene encoding pten (phosphatase and tensin homolog), enables RGCs to regenerate axons the full length of the optic nerve in mature mice; approximately half of these axons cross the chiasm, and a rare subset (∼1%) manages to enter the thalamus. Consistent with our previous findings, the axon-promoting effects of inflammation were shown to require the macrophage-derived growth factor Oncomodulin (Ocm). Elevation of cAMP increased the ability of Ocm to bind to its receptors in the inner retina and augmented inflammation-induced regeneration twofold. Inflammation combined with elevated cAMP and PTEN deletion increased activation of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase signaling pathways and augmented regeneration ∼10-fold over the level induced by either pten deletion or Zymosan alone. Thus, treatments that synergistically alter the intrinsic growth state of RGCs produce unprecedented levels of axon regeneration in the optic nerve, a CNS pathway long believed to be incapable of supporting such growth.
Figures



References
-
- Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. - PubMed
-
- Berry M, Carlile J, Hunter A, Tsang W, Rosensteil P, Rosustrel P, Sievers J. Optic nerve regeneration after intravitreal peripheral nerve implants: trajectories of axons regrowing through the optic chiasm into the optic tracts. J Neurocytol. 1999;28:721–741. - PubMed
-
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. - PubMed
-
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
