Regeneration of new neurons is preserved in aged vomeronasal epithelia
- PMID: 21084624
- PMCID: PMC3393108
- DOI: 10.1523/JNEUROSCI.4316-10.2010
Regeneration of new neurons is preserved in aged vomeronasal epithelia
Abstract
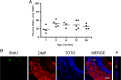
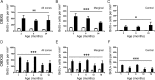
During normal and diseased aging, it is thought the capacity for tissue regeneration and repair in neuronal tissues diminishes. In the peripheral olfactory system, stem cell reservoirs permit regeneration of olfactory and vomeronasal sensory neurons, a unique capacity among neurons. Following injury, a large number of new neurons can be regenerated in a young animal. However, it is unknown whether this capacity for renewal exists in aged proliferative populations. Here, we report that neuronal replacement-associated proliferation continues in the vomeronasal organ of aged (18-24 months) mice. In addition, the potential for the aged stem cell to yield a mature neuron persisted at the same rate as that observed in young animals. Furthermore, the robust regenerative capacity to respond to both acute and sustained injury following olfactory bulbectomy remains intact even in very old animals. Hence, the neuronal epithelium lining the vomeronasal organ is unique in that it contains stem cells capable of generating functional neurons throughout life and in the aged animal in particular. This persistent regenerative capacity provides hope for neuronal replacement therapies in the aged nervous system.
Figures
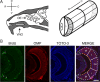
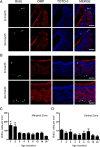
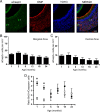
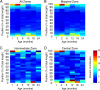

Similar articles
-
Gli3 Regulates Vomeronasal Neurogenesis, Olfactory Ensheathing Cell Formation, and GnRH-1 Neuronal Migration.J Neurosci. 2020 Jan 8;40(2):311-326. doi: 10.1523/JNEUROSCI.1977-19.2019. Epub 2019 Nov 25. J Neurosci. 2020. PMID: 31767679 Free PMC article.
-
Cell birth and survival following seasonal periods of cell proliferation in the chemosensory epithelia of red-backed salamanders, Plethodon cinereus.Brain Behav Evol. 2006;68(1):26-36. doi: 10.1159/000092311. Epub 2006 Mar 24. Brain Behav Evol. 2006. PMID: 16567929
-
Neurogenesis, migration, and apoptosis in the vomeronasal epithelium of adult mice.J Neurobiol. 2005 Jun;63(3):173-87. doi: 10.1002/neu.20128. J Neurobiol. 2005. PMID: 15729685
-
Regeneration of olfactory receptor cells.Ciba Found Symp. 1991;160:233-42; discussion 243-8. doi: 10.1002/9780470514122.ch12. Ciba Found Symp. 1991. PMID: 1752165 Review.
-
Pheromone detection by mammalian vomeronasal neurons.Microsc Res Tech. 2002 Aug 1;58(3):251-60. doi: 10.1002/jemt.10152. Microsc Res Tech. 2002. PMID: 12203702 Review.
Cited by
-
Age-dependent regional changes in the rostral migratory stream.Neurobiol Aging. 2013 Jul;34(7):1873-81. doi: 10.1016/j.neurobiolaging.2013.01.015. Epub 2013 Feb 15. Neurobiol Aging. 2013. PMID: 23419702 Free PMC article.
-
Injury in aged animals robustly activates quiescent olfactory neural stem cells.Front Neurosci. 2015 Oct 8;9:367. doi: 10.3389/fnins.2015.00367. eCollection 2015. Front Neurosci. 2015. PMID: 26500487 Free PMC article.
-
Molecular, cellular, and developmental organization of the mouse vomeronasal organ at single cell resolution.Elife. 2024 Dec 10;13:RP97356. doi: 10.7554/eLife.97356. Elife. 2024. PMID: 39656606 Free PMC article.
-
The phototransduction machinery in the rod outer segment has a strong efficacy gradient.Proc Natl Acad Sci U S A. 2015 May 19;112(20):E2715-24. doi: 10.1073/pnas.1423162112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941368 Free PMC article.
-
Aging in the olfactory system.Trends Neurosci. 2014 Feb;37(2):77-84. doi: 10.1016/j.tins.2013.11.004. Epub 2013 Dec 19. Trends Neurosci. 2014. PMID: 24361044 Free PMC article. Review.
References
-
- Bailey KJ, Maslov AY, Pruitt SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. - PubMed
-
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. - PubMed
-
- Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, Shou J, Wu HH. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58:176–188. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
