The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-beta-mediated antiviral activity in vitro and in vivo
- PMID: 21084628
- PMCID: PMC3040469
- DOI: 10.1189/jlb.0410216
The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-beta-mediated antiviral activity in vitro and in vivo
Abstract
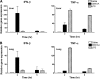
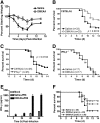
The 2009 outbreak of pandemic H1N1 influenza, increased drug resistance, and the significant delay in obtaining adequate numbers of vaccine doses have heightened awareness of the need to develop new antiviral drugs that can be used prophylactically or therapeutically. Previously, we showed that the experimental anti-tumor drug DMXAA potently induced IFN-β but relatively low TNF-α expression in vitro. This study confirms these findings in vivo and demonstrates further that DMXAA induces potent antiviral activity in vitro and in vivo. In vitro, DMXAA protected RAW 264.7 macrophage-like cells from VSV-induced cytotoxicity and moreover, inhibited replication of influenza, including the Tamiflu®-resistant H1N1 influenza A/Br strain, in MDCK cells. In vivo, DMXAA protected WT C57BL/6J but not IFN-β(-/-) mice from lethality induced by the mouse-adapted H1N1 PR8 influenza strain when administered before or after infection. Protection was accompanied by mitigation of weight loss, increased IFN-β mRNA and protein levels in the lung, and significant inhibition of viral replication in vivo early after DMXAA treatment. Collectively, this study provides data to support the use of DMXAA as a novel antiviral agent.
Figures

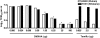
Comment in
-
Editorial: One small molecule: a new way to treat the flu?J Leukoc Biol. 2011 Mar;89(3):327-8. doi: 10.1189/jlb.1010580. J Leukoc Biol. 2011. PMID: 21357247 Free PMC article.
References
-
- Thompson W. W., Shay D. K., Weintraub E., Brammer L., Cox N., Anderson L. J., Fukuda K. (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 - PubMed
-
- Thompson W. W., Shay D. K., Weintraub E., Brammer L., Bridges C. B., Cox N. J., Fukuda K. (2004) Influenza-associated hospitalizations in the United States. JAMA 292, 1333–1340 - PubMed
-
- Moscona A. (2005) Neuraminidase inhibitors for influenza. N. Engl. J. Med. 353, 1363–1373 - PubMed
-
- Oxford J. S., Bossuyt S., Balasingam S., Mann A., Novelli P., Lambkin R. (2003) Treatment of epidemic and pandemic influenza with neuraminidase and M2 proton channel inhibitors. Clin. Microbiol. Infect. 9, 1–14 - PubMed
-
- Bright R. A., Medina M. J., Xu X., Perez-Oronoz G., Wallis T. R., Davis X. M., Povinelli L., Cox N. J., Klimov A. I. (2005) Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366, 1175–1181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

