Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding
- PMID: 21084633
- PMCID: PMC3000259
- DOI: 10.1073/pnas.1006370107
Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding
Abstract
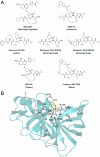
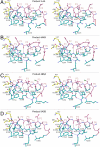
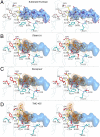
Hepatitis C virus infects an estimated 180 million people worldwide, prompting enormous efforts to develop inhibitors targeting the essential NS3/4A protease. Resistance against the most promising protease inhibitors, telaprevir, boceprevir, and ITMN-191, has emerged in clinical trials. In this study, crystal structures of the NS3/4A protease domain reveal that viral substrates bind to the protease active site in a conserved manner defining a consensus volume, or substrate envelope. Mutations that confer the most severe resistance in the clinic occur where the inhibitors protrude from the substrate envelope, as these changes selectively weaken inhibitor binding without compromising the binding of substrates. These findings suggest a general model for predicting the susceptibility of protease inhibitors to resistance: drugs designed to fit within the substrate envelope will be less susceptible to resistance, as mutations affecting inhibitor binding would simultaneously interfere with the recognition of viral substrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization. . Vaccine research: hepatitis C. In: Barnes E, editor. Hepatitis C Virus: Disease Burden. 2010. [March 15, 2010]. Available at http://www.who.int/vaccine_research/diseases/viral_cancers/en/index2.html.
-
- Major ME, Feinstone SM. The molecular virology of hepatitis C. Hepatology. 1997;25:1527–1538. - PubMed
-
- Qureshi SA. Hepatitis C virus—biology, host evasion strategies, and promising new therapies on the horizon. Med Res Rev. 2007;27:353–373. - PubMed
-
- Paolucci S, et al. Analysis of HIV drug-resistant quasispecies in plasma, peripheral blood mononuclear cells and viral isolates from treatment-naive and HAART patients. J Med Virol. 2001;65:207–217. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

