Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection
- PMID: 21084634
- PMCID: PMC3000291
- DOI: 10.1073/pnas.1011242107
Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection
Abstract
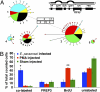
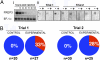
Invertebrates lack adaptive immune systems homologous to those of vertebrates, yet it is becoming increasingly clear that they can produce diversified antigen recognition molecules. We have previously noted that the snail Biomphalaria glabrata produces a secreted lectin, fibrinogen-related protein 3 (FREP3), unusual among invertebrate defense molecules because it is somatically diversified by gene conversion and point mutation. Here we implicate FREP3 in playing a central role in resistance to a major group of snail pathogens, digenetic trematodes. FREP3 is up-regulated in three models of resistance of B. glabrata to infection with Schistosoma mansoni or Echinostoma paraensei, and functions as an opsonin favoring phagocytosis by hemocytes. Knock-down of FREP3 in resistant snails using siRNA-mediated interference resulted in increased susceptibility to E. paraensei, providing a direct link between a gastropod immune molecule and resistance to trematodes. FREP3 up-regulation is also associated with heightened responsiveness following priming with attenuated digenetic trematodes (acquired resistance) in this model invertebrate immune system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Zhang SM, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. - PubMed
-
- Watson FL, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

