ILK mediates the effects of strain on intestinal epithelial wound closure
- PMID: 21084641
- PMCID: PMC3043633
- DOI: 10.1152/ajpcell.00273.2010
ILK mediates the effects of strain on intestinal epithelial wound closure
Abstract
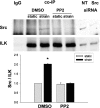
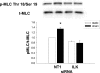
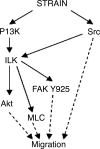
The intestinal epithelium is subjected to repetitive deformation during normal gut function by peristalsis and villous motility. Such repetitive strain promotes intestinal epithelial migration across fibronectin in vitro, but signaling mediators for this are poorly understood. We hypothesized that integrin-linked kinase (ILK) mediates strain-stimulated migration in intestinal epithelial cells cultured on fibronectin. ILK kinase activity increased rapidly 5 min after strain induction in both Caco-2 and intestinal epithelial cell-6 (IEC-6) cells. Wound closure in response to strain was reduced in ILK small interfering RNA (siRNA)-transfected Caco-2 cell monolayers when compared with control siRNA-transfected Caco-2 cells. Pharmacological blockade of phosphatidylinositol-3 kinase (PI3K) or Src or reducing Src by siRNA prevented strain activation of ILK. ILK coimmunoprecipitated with focal adhesion kinase (FAK), and this association was decreased by mutation of FAK Tyr925 but not FAK Tyr397. Strain induction of FAK Tyr925 phosphorylation but not FAK Tyr397 or FAK Tyr576 phosphorylation was blocked in ILK siRNA-transfected cells. ILK-Src association was stimulated by strain and was blocked by the Src inhibitor PP2. Finally, ILK reduction by siRNA inhibited strain-induced phosphorylation of myosin light chain and Akt. These results suggest a strain-dependent signaling pathway in which ILK association with FAK and Src mediates the subsequent downstream strain-induced motogenic response and suggest that ILK induction by repetitive deformation may contribute to recovery from mucosal injury and restoration of the mucosal barrier in patients with prolonged ileus. ILK may therefore be an important target for intervention to maintain the mucosa in such patients.
Figures






References
-
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure 10: 319–327, 2002 - PubMed
-
- Assi K, Mills J, Owen D, Ong C, St. Arnaud R, Dedhar S, Salh B. Integrin-linked kinase regulates cell proliferation and tumour growth in murine colitis-associated carcinogenesis. Gut 57: 931–940, 2008 - PubMed
-
- Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol 168: 476–488, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

