Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation
- PMID: 21084642
- PMCID: PMC3043627
- DOI: 10.1152/ajpcell.00172.2010
Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation
Abstract
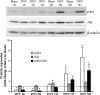
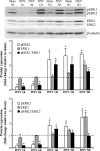
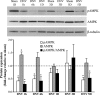
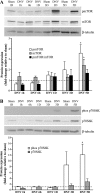
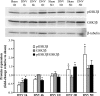
Unilateral denervation (DNV) of rat diaphragm muscle increases protein synthesis at 3 days after DNV (DNV-3D) and degradation at DNV-5D, such that net protein breakdown is evident by DNV-5D. On the basis of existing models of protein balance, we examined DNV-induced changes in Akt, AMP-activated protein kinase (AMPK), and ERK½ activation, which can lead to increased protein synthesis via mammalian target of rapamycin (mTOR)/p70S6 kinase (p70S6K), glycogen synthase kinase-3β (GSK3β), or eukaryotic initiation factor 4E (eIF4E), and increased protein degradation via forkhead box protein O (FoxO). Protein phosphorylation was measured using Western analyses through DNV-5D. Akt phosphorylation decreased at 1 h and 6 h after DNV compared with sham despite decreased AMPK phosphorylation. Both Akt and AMPK phosphorylation returned to sham levels by DNV-1D. Phosphorylation of their downstream effector mTOR (Ser2481) did not change at any time point after DNV, and phosphorylated p70S6K and eIF4E-binding protein 1 (4EBP1) increased only by DNV-5D. In contrast, ERK½ phosphorylation and its downstream effector eIF4E increased 1.7-fold at DNV-1D and phosphorylated GSK3β increased 1.5-fold at DNV-3D (P < 0.05 for both comparisons). Thus, following DNV there are differential effects on protein synthetic pathways with preferential activation of GSK3β and eIF4E over p70S6K. FoxO1 nuclear translocation occurred by DNV-1D, consistent with its role in increasing expression of atrogenes necessary for subsequent ubiquitin-proteasome activation evident by DNV-5D. On the basis of our results, increased protein synthesis following DNV is associated with changes in ERK½-dependent pathways, but protein degradation results from downregulation of Akt and nuclear translocation of FoxO1. No single trigger is responsible for protein balance following DNV. Protein balance in skeletal muscle depends on multiple synthetic/degradation pathways that should be studied in concert.
Figures



References
-
- Akoumianaki T, Kardassis D, Polioudaki H, Georgatos SD, Theodoropoulos PA. Nucleocytoplasmic shuttling of soluble tubulin in mammalian cells. J Cell Sci 122: 1111–1118, 2009 - PubMed
-
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000 - PubMed
-
- Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999 - PubMed
-
- Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL, Jr, Urban RJ. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383–E390, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

