mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish
- PMID: 21084707
- PMCID: PMC3056479
- DOI: 10.1182/blood-2010-10-314120
mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish
Abstract
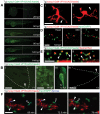
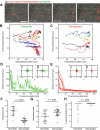
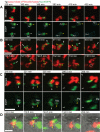
Macrophages and neutrophils play important roles during the innate immune response, phagocytosing invading microbes and delivering antimicrobial compounds to the site of injury. Functional analyses of the cellular innate immune response in zebrafish infection/inflammation models have been aided by transgenic lines with fluorophore-marked neutrophils. However, it has not been possible to study macrophage behaviors and neutrophil/macrophage interactions in vivo directly because there has been no macrophage-only reporter line. To remove this roadblock, a macrophage-specific marker was identified (mpeg1) and its promoter used in mpeg1-driven transgenes. mpeg1-driven transgenes are expressed in macrophage-lineage cells that do not express neutrophil-marking transgenes. Using these lines, the different dynamic behaviors of neutrophils and macrophages after wounding were compared side-by-side in compound transgenics. Macrophage/neutrophil interactions, such as phagocytosis of senescent neutrophils, were readily observed in real time. These zebrafish transgenes provide a new resource that will contribute to the fields of inflammation, infection, and leukocyte biology.
Figures

References
-
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. - PubMed
-
- van der Sar AM, Appelmelk BJ, Vandenbroucke-Grauls CM, Bitter W. A star with stripes: zebrafish as an infection model. Trends Microbiol. 2004;12(10):451–457. - PubMed
-
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978. - PubMed
-
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80(6):1281–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

