Sertoli cells dictate spermatogonial stem cell niches in the mouse testis
- PMID: 21084712
- PMCID: PMC3062034
- DOI: 10.1095/biolreprod.110.087320
Sertoli cells dictate spermatogonial stem cell niches in the mouse testis
Abstract
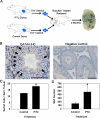
Sustained spermatogenesis in adult males relies on the activity of spermatogonial stem cells (SSCs). In general, tissue-specific stem cell populations such as SSCs are influenced by contributions of support cells that form niche microenvironments. Previous studies have provided indirect evidence that several somatic cell populations and the interstitial vasculature influence SSC functions, but an individual orchestrator of niches has not been described. In this study, functional transplantation of SSCs, in combination with experimental alteration of Sertoli cell content by polythiouracil (PTU)-induced transient hypothyroidism, was used to explore the relationship of Sertoli cells with SSCs in testes of adult mice. Transplantation of SSCs from PTU-treated donor mice into seminiferous tubules of normal recipient mice revealed a greater than 3-fold increase in SSCs compared to those from testes of non-PTU-treated donors. In addition, use of PTU-treated mice as recipients for transplantation of SSCs from normal donors revealed a greater than 3-fold increase of accessible niches compared to those of testes of non-PTU treated recipient mice with normal numbers of Sertoli cells. Importantly, the area of seminiferous tubules bordered by interstitial tissue and percentage of seminiferous tubules associated with blood vessels was found to be no different in testes of PTU-treated mice compared to controls, indicating that neither the vasculature nor interstitial support cell populations influenced the alteration of niche number. Collectively, these results provide direct evidence that Sertoli cells are the key somatic cell population dictating the number of SSCs and niches in mammalian testes.
Figures


Similar articles
-
Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period.J Anat. 2010 May;216(5):577-88. doi: 10.1111/j.1469-7580.2010.01219.x. J Anat. 2010. PMID: 20525087 Free PMC article.
-
The production of glial cell line-derived neurotrophic factor by human sertoli cells is substantially reduced in sertoli cell-only testes.Hum Reprod. 2017 May 1;32(5):1108-1117. doi: 10.1093/humrep/dex061. Hum Reprod. 2017. PMID: 28369535 Free PMC article.
-
Spermatogonial stem cell transplantation into nonablated mouse recipient testes.Stem Cell Reports. 2021 Jul 13;16(7):1832-1844. doi: 10.1016/j.stemcr.2021.05.013. Epub 2021 Jun 17. Stem Cell Reports. 2021. PMID: 34143973 Free PMC article.
-
The spermatogonial stem cell niche.Microsc Res Tech. 2009 Aug;72(8):580-5. doi: 10.1002/jemt.20699. Microsc Res Tech. 2009. PMID: 19263493 Review.
-
Spermatogonial stem cells: Progress and prospects.Asian J Androl. 2015 Sep-Oct;17(5):771-5. doi: 10.4103/1008-682X.154995. Asian J Androl. 2015. PMID: 25994650 Free PMC article. Review.
Cited by
-
Caprin controls follicle stem cell fate in the Drosophila ovary.PLoS One. 2012;7(4):e35365. doi: 10.1371/journal.pone.0035365. Epub 2012 Apr 6. PLoS One. 2012. PMID: 22493746 Free PMC article.
-
Elucidating the Transcriptional States of Spermatogenesis-Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq.Biomolecules. 2024 Jul 12;14(7):840. doi: 10.3390/biom14070840. Biomolecules. 2024. PMID: 39062554 Free PMC article. Review.
-
Differential Regulation of TLE3 in Sertoli Cells of the Testes during Postnatal Development.Cells. 2019 Sep 27;8(10):1156. doi: 10.3390/cells8101156. Cells. 2019. PMID: 31569653 Free PMC article.
-
Open niche regulation of mouse spermatogenic stem cells.Dev Growth Differ. 2018 Dec;60(9):542-552. doi: 10.1111/dgd.12574. Epub 2018 Nov 15. Dev Growth Differ. 2018. PMID: 30443901 Free PMC article. Review.
-
SETDB1 Regulates Porcine Spermatogonial Adhesion and Proliferation through Modulating MMP3/10 Transcription.Cells. 2022 Jan 22;11(3):370. doi: 10.3390/cells11030370. Cells. 2022. PMID: 35159180 Free PMC article.
References
-
- Sharpe R. Regulation of spermatogenesis. Knobil E, Neill JD. (Eds.), The Physiology of Reproduction, vol. 1, 2nd ed. New York: Raven Press; 1994: 1363 1434
-
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776 798 - PubMed
-
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004; 304: 1338 1340 - PubMed
-
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836 841 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

