Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity
- PMID: 21084750
- PMCID: PMC2993591
- DOI: 10.1172/JCI43274
Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity
Abstract
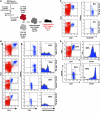
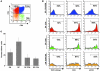
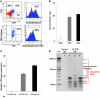
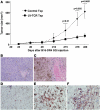
The development of effective cancer immunotherapies has been consistently hampered by several factors, including an inability to instigate long-term effective functional antitumor immunity. This is particularly true for immunotherapies that focus on the adoptive transfer of activated or genetically modified mature CD8+ T cells. In this study, we sought to alter and enhance long-term host immunity by genetically modifying, then transplanting, mouse HSCs. We first cloned a previously identified tumor-reactive HLA-DR4-restricted CD4+ TCR specific for the melanocyte differentiation antigen tyrosinase-related protein 1 (Tyrp1), then constructed both a high-expression lentivirus vector and a TCR-transgenic mouse expressing the genes encoding this TCR. Using these tools, we demonstrated that both mouse and human HSCs established durable, high-efficiency TCR gene transfer following long-term transplantation into lethally irradiated mice transgenic for HLA-DR4. Recipients of genetically modified mouse HSCs developed spontaneous autoimmune vitiligo that was associated with the presence of a Th1-polarized memory effector CD4+ T cell population that expressed the Tyrp1-specific TCR. Most importantly, large numbers of CD4+ T cells expressing the Tyrp1-specific TCR were detected in secondary HLA-DR4-transgenic transplant recipients, and these mice were able to destroy subcutaneously administered melanoma cells without the aid of vaccination, immune modulation, or cytokine administration. These results demonstrate the creation of what we believe to be a novel translational model of durable lentiviral gene transfer that results in long-term effective immunity.
Figures


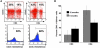




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

