Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice
- PMID: 21084751
- PMCID: PMC2993582
- DOI: 10.1172/JCI41624
Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice
Abstract
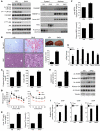
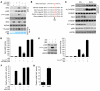
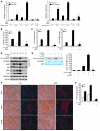
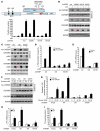
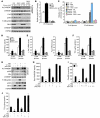
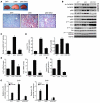
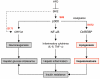
Obesity and type 2 diabetes are associated with increased lipogenesis in the liver. This results in fat accumulation in hepatocytes, a condition known as hepatic steatosis, which is a form of nonalcoholic fatty liver disease (NAFLD), the most common cause of liver dysfunction in the United States. Carbohydrate-responsive element-binding protein (ChREBP), a transcriptional activator of glycolytic and lipogenic genes, has emerged as a major player in the development of hepatic steatosis in mice. However, the molecular mechanisms enhancing its transcriptional activity remain largely unknown. In this study, we have identified the histone acetyltransferase (HAT) coactivator p300 and serine/threonine kinase salt-inducible kinase 2 (SIK2) as key upstream regulators of ChREBP activity. In cultured mouse hepatocytes, we showed that glucose-activated p300 acetylated ChREBP on Lys672 and increased its transcriptional activity by enhancing its recruitment to its target gene promoters. SIK2 inhibited p300 HAT activity by direct phosphorylation on Ser89, which in turn decreased ChREBP-mediated lipogenesis in hepatocytes and mice overexpressing SIK2. Moreover, both liver-specific SIK2 knockdown and p300 overexpression resulted in hepatic steatosis, insulin resistance, and inflammation, phenotypes reversed by SIK2/p300 co-overexpression. Finally, in mouse models of type 2 diabetes and obesity, low SIK2 activity was associated with increased p300 HAT activity, ChREBP hyperacetylation, and hepatic steatosis. Our findings suggest that inhibition of hepatic p300 activity may be beneficial for treating hepatic steatosis in obesity and type 2 diabetes and identify SIK2 activators and specific p300 inhibitors as potential targets for pharmaceutical intervention.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

