Cardiogenesis from human embryonic stem cells
- PMID: 21084757
- PMCID: PMC3938118
- DOI: 10.1253/circj.cj-10-0958
Cardiogenesis from human embryonic stem cells
Abstract
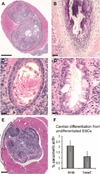
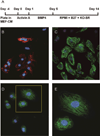
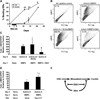
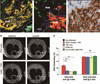
Over the past decade, the ability to culture and differentiate human embryonic stem cells (ESCs) has offered researchers a novel therapeutic that may, for the first time, repair regions of the damaged heart. Studies of cardiac development in lower organisms have led to identification of the transforming growth factor-β superfamily (eg, activin A and bone morphogenic protein 4) and the Wnt/β-catenin pathway as key inducers of mesoderm and cardiovascular differentiation. These factors act in a context-specific manner (eg, Wnt/β-catenin is required initially to form mesoderm but must be antagonized thereafter to make cardiac muscle). Different lines of ESCs produce different levels of agonists and antagonists for these pathways, but with careful optimization, highly enriched populations of immature cardiomyocytes can be generated. These cardiomyocytes survive transplantation to infarcted hearts of experimental animals, where they create new human myocardial tissue and improve heart function. The grafts generated by cell transplantation have been small, however, leading to an exploration of tissue engineering as an alternate strategy. Engineered tissue generated from preparations of human cardiomyocytes survives poorly after transplantation, most likely because of ischemia. Creation of pre-organized vascular networks in the tissue markedly enhances survival, with human capillaries anastomosed to the host coronary circulation. Thus, pathways controlling formation of the human cardiovascular system are emerging, yielding the building blocks for tissue regeneration that may address the root causes of heart failure.
Figures

Similar articles
-
Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16568-73. doi: 10.1073/pnas.0908381106. Epub 2009 Sep 17. Proc Natl Acad Sci U S A. 2009. PMID: 19805339 Free PMC article.
-
Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model.Circ Res. 2015 Sep 25;117(8):720-30. doi: 10.1161/CIRCRESAHA.115.306985. Epub 2015 Aug 19. Circ Res. 2015. PMID: 26291556 Free PMC article.
-
The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction.Biomaterials. 2013 May;34(16):4013-4026. doi: 10.1016/j.biomaterials.2013.02.022. Epub 2013 Mar 5. Biomaterials. 2013. PMID: 23465823
-
Stem cell engineering for treatment of heart diseases: potentials and challenges.Cell Biol Int. 2009 Mar;33(3):255-67. doi: 10.1016/j.cellbi.2008.11.009. Epub 2008 Dec 3. Cell Biol Int. 2009. PMID: 19084605 Review.
-
Stem cells in cardiac repair.Future Cardiol. 2011 Jan;7(1):99-117. doi: 10.2217/fca.10.109. Future Cardiol. 2011. PMID: 21174514 Review.
Cited by
-
Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview.Circ Res. 2012 Jul 20;111(3):344-58. doi: 10.1161/CIRCRESAHA.110.227512. Circ Res. 2012. PMID: 22821908 Free PMC article. Review.
-
Cardiac cell therapy: the next (re)generation.Stem Cell Rev Rep. 2011 Nov;7(4):1018-30. doi: 10.1007/s12015-011-9252-8. Stem Cell Rev Rep. 2011. PMID: 21437575
-
Incorporation of gold-coated microspheres into embryoid body of human embryonic stem cells for cardiomyogenic differentiation.Tissue Eng Part A. 2015 Jan;21(1-2):374-81. doi: 10.1089/ten.TEA.2014.0015. Epub 2014 Sep 8. Tissue Eng Part A. 2015. PMID: 25065511 Free PMC article.
-
The march of pluripotent stem cells in cardiovascular regenerative medicine.Stem Cell Res Ther. 2018 Jul 27;9(1):201. doi: 10.1186/s13287-018-0947-5. Stem Cell Res Ther. 2018. PMID: 30053890 Free PMC article. Review.
-
Silicon nanowire-induced maturation of cardiomyocytes derived from human induced pluripotent stem cells.Nano Lett. 2015 May 13;15(5):2765-72. doi: 10.1021/nl502227a. Epub 2015 Apr 7. Nano Lett. 2015. PMID: 25826336 Free PMC article.
References
-
- Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. - PubMed
-
- Mallory GK, White PD, Salcedo-Salga J. The speed of healing of myocardial infarction: A study of the pathologic anatomy in 72 cases. Am Heart J. 1939;18:647–671.
-
- Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. - PubMed
Publication types
MeSH terms
Grants and funding
- U01HL100405/HL/NHLBI NIH HHS/United States
- P01HL094374/HL/NHLBI NIH HHS/United States
- U01 HL100405/HL/NHLBI NIH HHS/United States
- T32 HL007828/HL/NHLBI NIH HHS/United States
- R01 HL064387/HL/NHLBI NIH HHS/United States
- R01HL084642/HL/NHLBI NIH HHS/United States
- P01GM81619/GM/NIGMS NIH HHS/United States
- P01 HL094374/HL/NHLBI NIH HHS/United States
- U24 DK076126/DK/NIDDK NIH HHS/United States
- R01 HL084642/HL/NHLBI NIH HHS/United States
- U24DK076126/DK/NIDDK NIH HHS/United States
- P01 GM081619/GM/NIGMS NIH HHS/United States
- R01HL64387/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

