Isolation of rotational isomers and developments derived therefrom
- PMID: 21084771
- PMCID: PMC3035921
- DOI: 10.2183/pjab.86.867
Isolation of rotational isomers and developments derived therefrom
Abstract
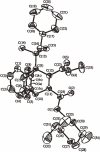
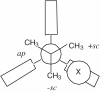
Isolation of rotational isomer models of ethane-type molecules is described. We could experimentally prove that, if rotational isomers whose molecular shape was chiral, the molecule could be optically active, even though it did not carry an asymmetric carbon atom. As an extension, other types of stereochemically fundamental and optically active molecules were isolated and their absolute stereochemistry was determined. One example is the model of meso-tartaric acid, for which optical inactivity had been attributed to internal compensation but is now explained as follows. On dissolution of meso-tartaric acid in a solvent, the molecule gives two kinds of conformers, one of which is a C(i) molecule and the other is a C(1) molecule. Although the latter is intrinsically optically active, the optical activity is cancelled by its enantiomer. The theory of internal compensation is recommended to be abandoned. As an extension to another area, some reactions of conformers are also discussed.
Figures

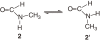
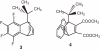

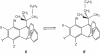


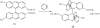



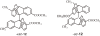

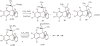
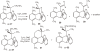
References
-
- Kemp J.D., Pitzer K.S. (1936) J. Chem. Phys. 4, 749
-
- Kemp J.D., Pitzer K.S. (1937) J. Am. Chem. Soc. 59, 276–279
-
- Mizushima, S. (1954) Molecular Structure and Internal Rotation. Academic, New York.
-
- Landolt, H. (1879) Das Optische Vermögen Organischer Substanzen. Vieweg und Sohn, Braunschweig, p. 32.
-
- Cahn R.S., Ingold C.K., Prelog V. (1956) Experientia 12, 81–94
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

