Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma
- PMID: 21084864
- PMCID: PMC3047099
- DOI: 10.4161/cbt.11.1.13835
Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma
Abstract
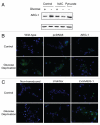
Glioblastomas continue to carry poor prognoses for patients despite advances in surgical, chemotherapeutic, and radiation regimens. One feature of glioblastoma associated with poor prognosis is the degree of hypoxia and expression levels of hypoxia-inducible factor-1 α (HIF-1α). HIF-1α expression allows metabolic adaptation to low oxygen availability, partly through upregulation of VEGF and increased tumor angiogenesis. Here, we demonstrate an induced level of astrocyte-elevated gene-1 (AEG-1) by hypoxia in glioblastoma cells. AEG-1 has the capacity to promote anchorage-independent growth and cooperates with Ha-ras in malignant transformation. In addition, AEG-1 was recently demonstrated to serve as an oncogene and can induce angiogenesis in glioblastoma. Results from in vitro studies show that hypoxic induction of AEG-1 is dependent on HIF-1α stabilization during hypoxia and that PI3K inhibition abrogates AEG-1 induction during hypoxia through loss of HIF-1α stability. Furthermore, we show that AEG-1 is induced by glucose deprivation and that prevention of intracellular reactive oxygen species (ROS) production prevents this induction. Additionally, AEG-1 knockdown results in increased ROS production and increased glucose deprivation-induced cytotoxicity. On the other hand, AEG-1 overexpression prevents ROS production and decreases glucose deprivation-induced cytotoxicity, indicating that AEG-1 induction is necessary for cells to survive this type of cell stress. These observations link AEG-1 overexpression in glioblastoma with hypoxia and glucose deprivation, and targeting these physiological pathways may lead to therapeutic advances in the treatment of glioblastoma in the future.
Figures




Comment in
-
Astrocyte-elevated gene-1 (AEG-1): Glioblastoma's helping hand during times of hypoxia and glucose deprivation?Cancer Biol Ther. 2011 Jan 1;11(1):40-2. doi: 10.4161/cbt.11.1.14139. Epub 2011 Jan 1. Cancer Biol Ther. 2011. PMID: 21150314 No abstract available.
References
-
- Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol. 2009;2:263–284. - PubMed
-
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. - PubMed
-
- Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
