Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases
- PMID: 21084962
- PMCID: PMC3168573
- DOI: 10.1097/PAS.0b013e3181f8ff05
Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases
Abstract
Background: There has been no uniform terminology for systematic analysis of mass-forming preinvasive neoplasms (which we term tumoral intraepithelial neoplasia) that occur specifically within the ampulla. Here, we provide a detailed analysis of these neoplasms, which we propose to refer to as intra-ampullary papillary-tubular neoplasm (IAPN).
Materials and methods: Three hundred and seventeen glandular neoplasms involving the ampulla were identified through a review of 1469 pancreatoduodenectomies and 11 ampullectomies. Eighty-two neoplasms characterized by substantial preinvasive exophytic component that grew almost exclusively (>75%) within the ampulla (in the ampullary channel or intra-ampullary portions of the very distal segments of the common bile duct or pancreatic duct) were analyzed.
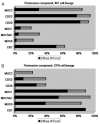
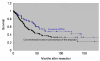
Results: (1) Clinical: The mean age was 64 years, male/female ratio was 2.4, and mean tumor size was 2.7 cm. (2) Pathology: The tumors had a mixture of both papillary and tubular growth (each constituting at least 25% of the lesion) in 57%; predominantly (>75%) papillary in 23%, and predominantly (>75%) tubular in 20%. High-grade dysplasia was present in 94% of cases, of which 39% showed focal (<25% of the lesion), 28% showed substantial (25% to 75%), and 27% showed extensive (>75%) high-grade dysplasia. In terms of cell-lineage morphology, 45% had a mixture of patterns. However, when evaluated with a forced-binary approach as intestinal (INT) versus gastric/pancreatobiliary (GPB) based on the predominant pattern, 74% were classified as INT and 26% as GPB. (3) Immunohistochemistry: Percent sensitivity/specificity of cell-lineage markers were, for INT phenotype: MUC2 85/78 and CDX2 94/61; and for GBP: MUC1 89/79, MUC5AC 95/69, and MUC6 83/76, respectively. Cytokeratin 7 and 20 were coexpressed in more than half. (4) Invasive carcinoma: In 64 cases (78%), there was an associated invasive carcinoma. Size of the tumor and amount of dysplasia correlated with the incidence of invasion. Invasive carcinoma was of INT-type in 58% and of pancreatobiliary-type in 42%. Cell lineage in the invasive component was the same as that of the preinvasive component in 84%. All discrepant cases were pancreatobiliary-type invasions, which occurred in INT-type preinvasive lesions. (5) OUTCOME: The overall survival of invasive cases were significantly worse than that of noninvasive ones (57% vs. 93%; P=0.01); and 3 years, 69% versus 100% (P=0.08); and 5 years, 45% versus 100% (P=0.07), respectively. When compared with 166 conventional invasive carcinomas of the ampullary region, invasive IAPNs had significantly better prognosis with a mean survival of 51 versus 31 months (P<0.001) and the 3-year survival of 69% versus 44% (P<0.01).
Conclusions: Tumoral intraepithelial neoplasia occurring within the ampulla are highly analogous to pancreatic or biliary intraductal papillary and tubular neoplasms as evidenced by their papillary and/or tubular growth, variable cell lineage, and spectrum of dysplastic change (adenoma-carcinoma sequence), and thus we propose to refer to these as IAPN. IAPNs are biologically indolent; noninvasive examples show an excellent prognosis, whereas those with invasion exhibit a malignant but nevertheless significantly better prognosis than typical invasive ampullary carcinomas unaccompanied by IAPNs. Twenty eight percent (64 of 230) of invasive carcinomas within the ampulla arise in association with IAPNs.
Figures








References
-
- Adsay NV, Klöppel G, Fukushima N, et al. Intraductal neoplasms of the exocrine pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. World Health Organization Classification of Tumours of the Digestive System. WHO Press; Geneva: in press.
-
- Abraham SC, Lee JH, Boitnott JK, et al. Microsatellite instability in intraductal papillary neoplasms of the biliary tract. Mod Pathol. 2002;15:1309–1317. - PubMed
-
- Abraham SC, Lee JH, Hruban RH, et al. Molecular and immunohistochemical analysis of intraductal papillary neoplasms of the biliary tract. Hum Pathol. 2003;34:902–910. - PubMed
-
- Adsay NV, Klimstra DS. Benign and malignant tumors of the gallbladder and extrahepatic biliary tract. In: Odze RD, Goldblum JR, editors. Surgical Pathology of the GI tract, Liver, Biliary Tract, and Pancreas. Elsevier; Philadelphia: 2009. pp. 845–875.
-
- Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

