Acid sensing by the Drosophila olfactory system
- PMID: 21085119
- PMCID: PMC3105465
- DOI: 10.1038/nature09537
Acid sensing by the Drosophila olfactory system
Abstract
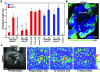
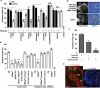
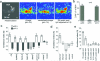
The odour of acids has a distinct quality that is perceived as sharp, pungent and often irritating. How acidity is sensed and translated into an appropriate behavioural response is poorly understood. Here we describe a functionally segregated population of olfactory sensory neurons in the fruitfly, Drosophila melanogaster, that are highly selective for acidity. These olfactory sensory neurons express IR64a, a member of the recently identified ionotropic receptor (IR) family of putative olfactory receptors. In vivo calcium imaging showed that IR64a+ neurons projecting to the DC4 glomerulus in the antennal lobe are specifically activated by acids. Flies in which the function of IR64a+ neurons or the IR64a gene is disrupted had defects in acid-evoked physiological and behavioural responses, but their responses to non-acidic odorants remained unaffected. Furthermore, artificial stimulation of IR64a+ neurons elicited avoidance responses. Taken together, these results identify cellular and molecular substrates for acid detection in the Drosophila olfactory system and support a labelled-line mode of acidity coding at the periphery.
Figures


References
-
- Dravnieks A. Odor quality: semantically generated multidimensional profiles are stable. Science. 1982;218(4574):799–801. - PubMed
-
- Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36(3):463–474. - PubMed
-
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112(2):271–282. - PubMed
-
- Kellogg FE. Water vapour and carbon dioxide receptors in Aedes aegypti. J Insect Physiol. 1970;16(1):99–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

