2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members
- PMID: 21085181
- PMCID: PMC3058805
- DOI: 10.1038/nature09489
2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members
Abstract
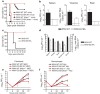
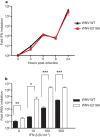
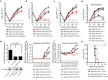
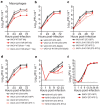
Cellular messenger RNA (mRNA) of higher eukaryotes and many viral RNAs are methylated at the N-7 and 2'-O positions of the 5' guanosine cap by specific nuclear and cytoplasmic methyltransferases (MTases), respectively. Whereas N-7 methylation is essential for RNA translation and stability, the function of 2'-O methylation has remained uncertain since its discovery 35 years ago. Here we show that a West Nile virus (WNV) mutant (E218A) that lacks 2'-O MTase activity was attenuated in wild-type primary cells and mice but was pathogenic in the absence of type I interferon (IFN) signalling. 2'-O methylation of viral RNA did not affect IFN induction in WNV-infected fibroblasts but instead modulated the antiviral effects of IFN-induced proteins with tetratricopeptide repeats (IFIT), which are interferon-stimulated genes (ISGs) implicated in regulation of protein translation. Poxvirus and coronavirus mutants that lacked 2'-O MTase activity similarly showed enhanced sensitivity to the antiviral actions of IFN and, specifically, IFIT proteins. Our results demonstrate that the 2'-O methylation of the 5' cap of viral RNA functions to subvert innate host antiviral responses through escape of IFIT-mediated suppression, and suggest an evolutionary explanation for 2'-O methylation of cellular mRNA: to distinguish self from non-self RNA. Differential methylation of cytoplasmic RNA probably serves as an example for pattern recognition and restriction of propagation of foreign viral RNA in host cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Muthukrishnan S, Moss B, Cooper JA, Maxwell ES. Influence of 5′-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J. Biol. Chem. 1978;253:1710–1715. - PubMed
-
- Langberg SR, Moss B. Post-transcriptional modifications of mRNA. Purification and characterization of cap I and cap II RNA (nucleoside-2′-)-methyltransferases from HeLa cells. J. Biol. Chem. 1981;256:10054–10060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

