Adaptive immunity against Leishmania nucleoside hydrolase maps its c-terminal domain as the target of the CD4+ T cell-driven protective response
- PMID: 21085470
- PMCID: PMC2976684
- DOI: 10.1371/journal.pntd.0000866
Adaptive immunity against Leishmania nucleoside hydrolase maps its c-terminal domain as the target of the CD4+ T cell-driven protective response
Abstract
Nucleoside hydrolases (NHs) show homology among parasite protozoa, fungi and bacteria. They are vital protagonists in the establishment of early infection and, therefore, are excellent candidates for the pathogen recognition by adaptive immune responses. Immune protection against NHs would prevent disease at the early infection of several pathogens. We have identified the domain of the NH of L. donovani (NH36) responsible for its immunogenicity and protective efficacy against murine visceral leishmaniasis (VL). Using recombinant generated peptides covering the whole NH36 sequence and saponin we demonstrate that protection against L. chagasi is related to its C-terminal domain (amino-acids 199-314) and is mediated mainly by a CD4+ T cell driven response with a lower contribution of CD8+ T cells. Immunization with this peptide exceeds in 36.73±12.33% the protective response induced by the cognate NH36 protein. Increases in IgM, IgG2a, IgG1 and IgG2b antibodies, CD4+ T cell proportions, IFN-γ secretion, ratios of IFN-γ/IL-10 producing CD4+ and CD8+ T cells and percents of antibody binding inhibition by synthetic predicted epitopes were detected in F3 vaccinated mice. The increases in DTH and in ratios of TNFα/IL-10 CD4+ producing cells were however the strong correlates of protection which was confirmed by in vivo depletion with monoclonal antibodies, algorithm predicted CD4 and CD8 epitopes and a pronounced decrease in parasite load (90.5-88.23%; p = 0.011) that was long-lasting. No decrease in parasite load was detected after vaccination with the N-domain of NH36, in spite of the induction of IFN-γ/IL-10 expression by CD4+ T cells after challenge. Both peptides reduced the size of footpad lesions, but only the C-domain reduced the parasite load of mice challenged with L. amazonensis. The identification of the target of the immune response to NH36 represents a basis for the rationale development of a bivalent vaccine against leishmaniasis and for multivalent vaccines against NHs-dependent pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

 p<0.05 different from F1sap vaccine; ○ p<0.05 different from the F2sap vaccine; ◆ p<0.05 different from NH36sap vaccine;
p<0.05 different from F1sap vaccine; ○ p<0.05 different from the F2sap vaccine; ◆ p<0.05 different from NH36sap vaccine;  p<0.05 different from all other vaccines.
p<0.05 different from all other vaccines.

 the F1, ○ the F2 or
the F1, ○ the F2 or  from all the other vaccines.
from all the other vaccines.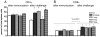

 from F1sap, ○ from F2sap, and ◆ from the NH36sap vaccine.
from F1sap, ○ from F2sap, and ◆ from the NH36sap vaccine.

 from the F1sap and ○ from the F2sap vaccines. The mean averages of LDU values are: 1632.64 (sal); 1027.50 (NH36sap); 1806.49 (F1sap); 1469.91 (F2sap) and 192.14 (F3sap).
from the F1sap and ○ from the F2sap vaccines. The mean averages of LDU values are: 1632.64 (sal); 1027.50 (NH36sap); 1806.49 (F1sap); 1469.91 (F2sap) and 192.14 (F3sap).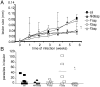

Similar articles
-
Nucleoside Hydrolase NH 36: A Vital Enzyme for the Leishmania Genus in the Development of T-Cell Epitope Cross-Protective Vaccines.Front Immunol. 2019 Apr 16;10:813. doi: 10.3389/fimmu.2019.00813. eCollection 2019. Front Immunol. 2019. PMID: 31040850 Free PMC article. Review.
-
The F1F3 recombinant chimera induced higher vaccine efficacy than its independent F1 and F3 components against Leishmania (L.) infantum chagasi mice infection.Front Immunol. 2025 Jul 1;16:1598755. doi: 10.3389/fimmu.2025.1598755. eCollection 2025. Front Immunol. 2025. PMID: 40666521 Free PMC article.
-
Cross-Protective Immunity to Leishmania amazonensis is Mediated by CD4+ and CD8+ Epitopes of Leishmania donovani Nucleoside Hydrolase Terminal Domains.Front Immunol. 2014 May 1;5:189. doi: 10.3389/fimmu.2014.00189. eCollection 2014. Front Immunol. 2014. PMID: 24822054 Free PMC article.
-
The F1F3 Recombinant Chimera of Leishmania donovani-Nucleoside Hydrolase (NH36) and Its Epitopes Induce Cross-Protection Against Leishmania (V.) braziliensis Infection in Mice.Front Immunol. 2019 Apr 9;10:724. doi: 10.3389/fimmu.2019.00724. eCollection 2019. Front Immunol. 2019. PMID: 31024556 Free PMC article.
-
Making an anti-amastigote vaccine for visceral leishmaniasis: rational, update and perspectives.Curr Opin Microbiol. 2012 Aug;15(4):476-85. doi: 10.1016/j.mib.2012.05.002. Epub 2012 Jun 13. Curr Opin Microbiol. 2012. PMID: 22698479 Review.
Cited by
-
Nucleoside Hydrolase NH 36: A Vital Enzyme for the Leishmania Genus in the Development of T-Cell Epitope Cross-Protective Vaccines.Front Immunol. 2019 Apr 16;10:813. doi: 10.3389/fimmu.2019.00813. eCollection 2019. Front Immunol. 2019. PMID: 31040850 Free PMC article. Review.
-
Leishmania donovani Nucleoside Hydrolase Terminal Domains in Cross-Protective Immunotherapy Against Leishmania amazonensis Murine Infection.Front Immunol. 2014 Jun 11;5:273. doi: 10.3389/fimmu.2014.00273. eCollection 2014. Front Immunol. 2014. PMID: 24966857 Free PMC article.
-
Immucillins ImmA and ImmH Are Effective and Non-toxic in the Treatment of Experimental Visceral Leishmaniasis.PLoS Negl Trop Dis. 2015 Dec 23;9(12):e0004297. doi: 10.1371/journal.pntd.0004297. eCollection 2015 Dec. PLoS Negl Trop Dis. 2015. PMID: 26701750 Free PMC article.
-
Vaccines for canine leishmaniasis.Front Immunol. 2012 Apr 17;3:69. doi: 10.3389/fimmu.2012.00069. eCollection 2012. Front Immunol. 2012. PMID: 22566950 Free PMC article.
-
The Delay in the Licensing of Protozoal Vaccines: A Comparative History.Front Immunol. 2020 Mar 6;11:204. doi: 10.3389/fimmu.2020.00204. eCollection 2020. Front Immunol. 2020. PMID: 32210953 Free PMC article. Review.
References
-
- Lukes J, Mauricio IL, Schönian G, Dujardin JC, Soteriadou K, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci U S A. 2007;104:9375–9380. doi: 10.1073/pnas.0703678104. - DOI - PMC - PubMed
-
- Mauricio IL, Yeo M, Baghaei M, Doto D, Pratlong F. Towards multilocus sequence typing of the Leishmania donovani complex: resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD). Int J Parasitol. 2006;36:757–769. doi: 10.1016/j.ijpara.2006.03.006. - DOI - PubMed
-
- Gopaul DN, Meyer SL, Degano M, Sacchettini JC, Schramm VL. Inosine-uridine nucleoside hydrolase from Crithidia fasciculata. Genetic characterization, crystallization, and identification of histidine 241 as a catalytic site residue. Biochemistry. 1996;35:5963–5970. doi: 10.1021/bi952998u. - DOI - PubMed
-
- Pellé R, Schramm VL, Parkin DW. Molecular cloning and expression of a purine-specific N-ribohydrolase from Trypanosoma brucei brucei. Sequence, expression and molecular analysis. J Biol Chem. 1998;273:2118–2126. doi: 10.1074/jbc.273.4.2118. - DOI - PubMed
-
- Miller RL, Sabourin CLK, Krenitsky TA, Berens RL, Marr JJ. Nucleoside Hydrolase from Trypanosoma cruzi. J Biol Chem. 1984;259:5073–5077. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

