Early acquisition of neural crest competence during hESCs neuralization
- PMID: 21085480
- PMCID: PMC2976694
- DOI: 10.1371/journal.pone.0013890
Early acquisition of neural crest competence during hESCs neuralization
Abstract
Background: Neural crest stem cells (NCSCs) are a transient multipotent embryonic cell population that represents a defining characteristic of vertebrates. The neural crest (NC) gives rise to many derivatives including the neurons and glia of the sensory and autonomic ganglia of the peripheral nervous system, enteric neurons and glia, melanocytes, and the cartilaginous, bony and connective tissue of the craniofacial skeleton, cephalic neuroendocrine organs, and some heart vessels.
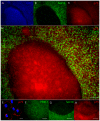
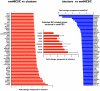
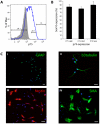
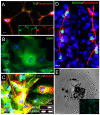
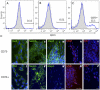
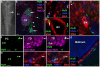
Methodology/principal findings: We present evidence that neural crest (NC) competence can be acquired very early when human embryonic stem cells (hESCs) are selectively neuralized towards dorsal neuroepithelium in the absence of feeder cells in fully defined conditions. When hESC-derived neurospheres are plated on fibronectin, some cells emigrate onto the substrate. These early migratory Neural Crest Stem Cells (emNCSCs) uniformly upregulate Sox10 and vimentin, downregulate N-cadherin, and remodel F-actin, consistent with a transition from neuroepithelium to a mesenchymal NC cell. Over 13% of emNCSCs upregulate CD73, a marker of mesenchymal lineage characteristic of cephalic NC and connexin 43, found on early migratory NC cells. We demonstrated that emNCSCs give rise in vitro to all NC lineages, are multipotent on clonal level, and appropriately respond to developmental factors. We suggest that human emNCSC resemble cephalic NC described in model organisms. Ex vivo emNCSCs can differentiate into neurons in Ret.k(-) mouse embryonic gut tissue cultures and transplanted emNCSCs incorporate into NC-derived structures but not CNS tissues in chick embryos.
Conclusions/significance: These findings will provide a framework for further studying early human NC development including the epithelial to mesenchymal transition during NC delamination.
Conflict of interest statement
Figures






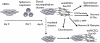
Similar articles
-
Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells.Stem Cells Transl Med. 2012 Apr;1(4):266-78. doi: 10.5966/sctm.2011-0042. Epub 2012 Apr 17. Stem Cells Transl Med. 2012. PMID: 23197806 Free PMC article.
-
HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia.J Neurosci. 2014 Apr 23;34(17):6112-22. doi: 10.1523/JNEUROSCI.5212-13.2014. J Neurosci. 2014. PMID: 24760871 Free PMC article.
-
The issue of the multipotency of the neural crest cells.Dev Biol. 2018 Dec 1;444 Suppl 1:S47-S59. doi: 10.1016/j.ydbio.2018.03.024. Epub 2018 Mar 31. Dev Biol. 2018. PMID: 29614271 Review.
-
Environmental factors unveil dormant developmental capacities in multipotent progenitors of the trunk neural crest.Dev Biol. 2013 Dec 1;384(1):13-25. doi: 10.1016/j.ydbio.2013.09.030. Epub 2013 Oct 4. Dev Biol. 2013. PMID: 24099925
-
The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities.Cell Cycle. 2010 Jan 15;9(2):238-49. doi: 10.4161/cc.9.2.10491. Epub 2010 Jan 26. Cell Cycle. 2010. PMID: 20037475 Review.
Cited by
-
Crestospheres: Long-Term Maintenance of Multipotent, Premigratory Neural Crest Stem Cells.Stem Cell Reports. 2015 Oct 13;5(4):499-507. doi: 10.1016/j.stemcr.2015.08.017. Epub 2015 Oct 1. Stem Cell Reports. 2015. PMID: 26441305 Free PMC article.
-
In vitro endothelial cell migration from limbal edge-modified Quarter-DMEK grafts.PLoS One. 2019 Nov 20;14(11):e0225462. doi: 10.1371/journal.pone.0225462. eCollection 2019. PLoS One. 2019. PMID: 31747441 Free PMC article.
-
Modeling physiological and pathological human neurogenesis in the dish.Front Neurosci. 2014 Jul 24;8:183. doi: 10.3389/fnins.2014.00183. eCollection 2014. Front Neurosci. 2014. PMID: 25104921 Free PMC article. Review.
-
WNT/β-catenin modulates the axial identity of embryonic stem cell-derived human neural crest.Development. 2019 Aug 29;146(16):dev175604. doi: 10.1242/dev.175604. Development. 2019. PMID: 31399472 Free PMC article.
-
Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells.Stem Cells Transl Med. 2012 Apr;1(4):266-78. doi: 10.5966/sctm.2011-0042. Epub 2012 Apr 17. Stem Cells Transl Med. 2012. PMID: 23197806 Free PMC article.
References
-
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. - PubMed
-
- Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. - PubMed
-
- LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

