Role of the epigenetic regulator HP1γ in the control of embryonic stem cell properties
- PMID: 21085495
- PMCID: PMC2981578
- DOI: 10.1371/journal.pone.0015507
Role of the epigenetic regulator HP1γ in the control of embryonic stem cell properties
Abstract
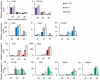
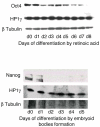
The unique properties of embryonic stem cells (ESC) rely on long-lasting self-renewal and their ability to switch in all adult cell type programs. Recent advances have shown that regulations at the chromatin level sustain both ESC properties along with transcription factors. We have focused our interest on the epigenetic modulator HP1γ (Heterochromatin Protein 1, isoform γ) that binds histones H3 methylated at lysine 9 (meH3K9) and is highly plastic in its distribution and association with the transcriptional regulation of specific genes during cell fate transitions. These characteristics of HP1γ make it a good candidate to sustain the ESC flexibility required for rapid program changes during differentiation. Using RNA interference, we describe the functional role of HP1γ in mouse ESC. The analysis of HP1γ deprived cells in proliferative and in various differentiating conditions was performed combining functional assays with molecular approaches (RT-qPCR, microarray). We show that HP1γ deprivation slows down the cell cycle of ESC and decreases their resistance to differentiating conditions, rendering the cells poised to differentiate. In addition, HP1γ depletion hampers the differentiation to the endoderm as compared with the differentiation to the neurectoderm or the mesoderm. Altogether, our results reveal the role of HP1γ in ESC self-renewal and in the balance between the pluripotent and the differentiation programs.
Conflict of interest statement
Figures


References
-
- O'Shea KS. Self-renewal vs. differentiation of mouse embryonic stem cells. Biol Reprod. 2004;71:1755–1765. - PubMed
-
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. - PubMed
-
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
-
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

