Towards racemizable chiral organogelators
- PMID: 21085504
- PMCID: PMC2981819
- DOI: 10.3762/bjoc.6.107
Towards racemizable chiral organogelators
Abstract
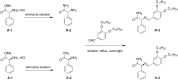
A chiral organogelator has been synthesized that can be racemized and self-assembled in apolar solvents whilst at higher concentrations organogels are formed. Field emission scanning and transmission electron microscopy revealed the formation of bundle fibrils that are able to gelate the solvent. ¹H NMR studies showed hydrogen-bond interactions between the peptide head groups of neighbouring organogelator molecules. The enantiomerically pure organogelator can be racemized by the base DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) as was evident from chiral high-performance liquid chromatography analysis.
Keywords: chirality; organogels; racemization; self-assembly.
Figures




Similar articles
-
Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups.Molecules. 2019 Jan 19;24(2):352. doi: 10.3390/molecules24020352. Molecules. 2019. PMID: 30669453 Free PMC article.
-
Organogelation by polymer organogelators with a L-lysine derivative: formation of a three-dimensional network consisting of supramolecular and conventional polymers.Chemistry. 2007;13(29):8193-200. doi: 10.1002/chem.200700146. Chemistry. 2007. PMID: 17639539
-
Using fourier transform infrared spectroscopy to examine structure in bisurea organogels.Appl Spectrosc. 2007 Apr;61(4):379-87. doi: 10.1366/000370207780466145. Appl Spectrosc. 2007. PMID: 17456256
-
Self-Assembly of Azobenzene Derivatives into Organogels and Photoresponsive Liquid Crystals.Chem Asian J. 2018 May 4;13(9):1173-1179. doi: 10.1002/asia.201800019. Epub 2018 Mar 30. Chem Asian J. 2018. PMID: 29453904
-
Accelerated Multiphosphorylated Peptide Synthesis.Org Process Res Dev. 2022 Aug 19;26(8):2492-2497. doi: 10.1021/acs.oprd.2c00164. Epub 2022 Jul 12. Org Process Res Dev. 2022. PMID: 36032360 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
