APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells
- PMID: 21085580
- PMCID: PMC2981559
- DOI: 10.1371/journal.pone.0013989
APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells
Abstract
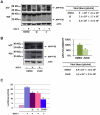
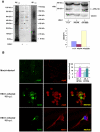
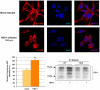
Lifelong latent infections of the trigeminal ganglion by the neurotropic herpes simplex virus type 1 (HSV-1) are characterized by periodic reactivation. During these episodes, newly produced virions may also reach the central nervous system (CNS), causing productive but generally asymptomatic infections. Epidemiological and experimental findings suggest that HSV-1 might contribute to the pathogenesis of Alzheimer's disease (AD). This multifactorial neurodegenerative disorder is related to an overproduction of amyloid beta (Aβ) and other neurotoxic peptides, which occurs during amyloidogenic endoproteolytic processing of the transmembrane amyloid precursor protein (APP). The aim of our study was to identify the effects of productive HSV-1 infection on APP processing in neuronal cells. We found that infection of SH-SY5Y human neuroblastoma cells and rat cortical neurons is followed by multiple cleavages of APP, which result in the intra- and/or extra-cellular accumulation of various neurotoxic species. These include: i) APP fragments (APP-Fs) of 35 and 45 kDa (APP-F35 and APP-F45) that comprise portions of Aβ; ii) N-terminal APP-Fs that are secreted; iii) intracellular C-terminal APP-Fs; and iv) Aβ(1-40) and Aβ(1-42). Western blot analysis of infected-cell lysates treated with formic acid suggests that APP-F35 may be an Aβ oligomer. The multiple cleavages of APP that occur in infected cells are produced in part by known components of the amyloidogenic APP processing pathway, i.e., host-cell β-secretase, γ-secretase, and caspase-3-like enzymes. These findings demonstrate that HSV-1 infection of neuronal cells can generate multiple APP fragments with well-documented neurotoxic potentials. It is tempting to speculate that intra- and extracellular accumulation of these species in the CNS resulting from repeated HSV-1 reactivation could, in the presence of other risk factors, play a co-factorial role in the development of AD.
Conflict of interest statement
Figures


References
-
- Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J Med Virol. 1991;33:224–227. - PubMed
-
- Itabashi S, Arai H, Matsui T, Higuchi S, Sasaki H. Herpes simplex virus and risk of Alzheimer’s disease. Lancet. 1997;349:1102. - PubMed
-
- Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J Med Virol. 2005;75:300–306. - PubMed
-
- Kastrukoff L, Hamada T, Schumacher U, Long C, Doherty PC, et al. Central nervous system infection and immune response in mice inoculated into the lip with herpes simplex virus type 1. J Neuroimmunol. 1982;2:295–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

