Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice
- PMID: 21085588
- PMCID: PMC2981567
- DOI: 10.1371/journal.pone.0013993
Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice
Abstract
Background: Several brain disturbances have been described in association to type 1 diabetes in humans. In animal models, hippocampal pathological changes were reported together with cognitive deficits. The exposure to a variety of environmental stimuli during a certain period of time is able to prevent brain alterations and to improve learning and memory in conditions like stress, aging and neurodegenerative processes.
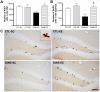
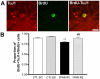
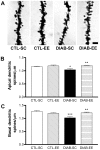
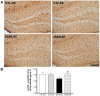
Methodology/principal findings: We explored the modulation of hippocampal alterations in streptozotocin-induced type 1 diabetic mice by environmental enrichment. In diabetic mice housed in standard conditions we found a reduction of adult neurogenesis in the dentate gyrus, decreased dendritic complexity in CA1 neurons and a smaller vascular fractional area in the dentate gyrus, compared with control animals in the same housing condition. A short exposure -10 days- to an enriched environment was able to enhance proliferation, survival and dendritic arborization of newborn neurons, to recover dendritic tree length and spine density of pyramidal CA1 neurons and to increase the vascular network of the dentate gyrus in diabetic animals.
Conclusions/significance: The environmental complexity seems to constitute a strong stimulator competent to rescue the diabetic brain from neurodegenerative progression.
Conflict of interest statement
Figures



References
-
- Biessels GJ, Gispen WH. The impact of diabetes on cognition: what can be learned from rodent models? Neurobiol Aging. 2005;26(Suppl 1):36–41. - PubMed
-
- McCall A. The impact of diabetes on the CNS. Diabetes. 1992;41:557–570. - PubMed
-
- Kamal A, Biessels GJ, Urban I, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Neuroscience. 1999;90:737–745. - PubMed
-
- Saravia F, Revsin Y, Gonzalez-Deniselle MS, Roig P, Lima A, et al. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Research. 2002;957:345–353. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

