Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate
- PMID: 21085591
- PMCID: PMC2981570
- DOI: 10.1371/journal.pone.0013983
Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate
Abstract
Background: We conducted a Phase I randomized, dose-escalation, route-comparison trial of MVA-CMDR, a candidate HIV-1 vaccine based on a recombinant modified vaccinia Ankara viral vector expressing HIV-1 genes env/gag/pol. The HIV sequences were derived from circulating recombinant form CRF01_AE, which predominates in Thailand. The objective was to evaluate safety and immunogenicity of MVA-CMDR in human volunteers in the US and Thailand.
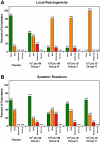
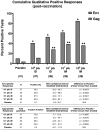
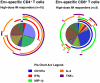
Methodology/principal findings: MVA-CMDR or placebo was administered intra-muscularly (IM; 10(7) or 10(8) pfu) or intradermally (ID; 10(6) or 10(7) pfu) at months 0, 1 and 3, to 48 healthy volunteers at low risk for HIV-1 infection. Twelve volunteers in each dosage group were randomized to receive MVA-CMDR or placebo (10∶2). Volunteers were actively monitored for local and systemic reactogenicity and adverse events post vaccination. Cellular immunogenicity was assessed by a validated IFNγ Elispot assay, an intracellular cytokine staining assay, lymphocyte proliferation and a (51)Cr-release assay. Humoral immunogenicity was assessed by ADCC for gp120 and binding antibody ELISAs for gp120 and p24. MVA-CMDR was safe and well tolerated with no vaccine related serious adverse events. Cell-mediated immune responses were: (i) moderate in magnitude (median IFNγ Elispot of 78 SFC/10(6) PBMC at 10(8) pfu IM), but high in response rate (70% (51)Cr-release positive; 90% Elispot positive; 100% ICS positive, at 10(8) pfu IM); (ii) predominantly HIV Env-specific CD4(+) T cells, with a high proliferative capacity and durable for at least 6 months (100% LPA response rate by the IM route); (iv) dose- and route-dependent with 10(8) pfu IM being the most immunogenic treatment. Binding antibodies against gp120 and p24 were detectable in all vaccination groups with ADCC capacity detectable at the highest dose (40% positive at 10(8) pfu IM).
Conclusions/significance: MVA-CMDR delivered both intramuscularly and intradermally was safe, well-tolerated and elicited durable cell-mediated and humoral immune responses.
Trial registration: ClinicalTrials.gov NCT00376090.
Conflict of interest statement
Figures


References
-
- UNAIDS. AIDS: Epidemic Update: November 2009. 2009. UNAIDS/09.36E/JC1700E.
-
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

