HIV-1 induces DCIR expression in CD4+ T cells
- PMID: 21085612
- PMCID: PMC2978727
- DOI: 10.1371/journal.ppat.1001188
HIV-1 induces DCIR expression in CD4+ T cells
Abstract
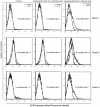
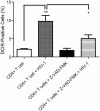
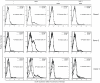
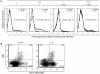
The C-type lectin receptor DCIR, which has been shown very recently to act as an attachment factor for HIV-1 in dendritic cells, is expressed predominantly on antigen-presenting cells. However, this concept was recently challenged by the discovery that DCIR can also be detected in CD4(+) T cells found in the synovial tissue from rheumatoid arthritis (RA) patients. Given that RA and HIV-1 infections share common features such as a chronic inflammatory condition and polyclonal immune hyperactivation status, we hypothesized that HIV-1 could promote DCIR expression in CD4(+) T cells. We report here that HIV-1 drives DCIR expression in human primary CD4(+) T cells isolated from patients (from both aviremic/treated and viremic/treatment naive persons) and cells acutely infected in vitro (seen in both virus-infected and uninfected cells). Soluble factors produced by virus-infected cells are responsible for the noticed DCIR up-regulation on uninfected cells. Infection studies with Vpr- or Nef-deleted viruses revealed that these two viral genes are not contributing to the mechanism of DCIR induction that is seen following acute infection of CD4(+) T cells with HIV-1. Moreover, we report that DCIR is linked to caspase-dependent (induced by a mitochondria-mediated generation of free radicals) and -independent intrinsic apoptotic pathways (involving the death effector AIF). Finally, we demonstrate that the higher surface expression of DCIR in CD4(+) T cells is accompanied by an enhancement of virus attachment/entry, replication and transfer. This study shows for the first time that HIV-1 induces DCIR membrane expression in CD4(+) T cells, a process that might promote virus dissemination throughout the infected organism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




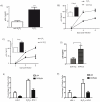
Similar articles
-
Dendritic cell immunoreceptor is a new target for anti-AIDS drug development: identification of DCIR/HIV-1 inhibitors.PLoS One. 2013 Jul 9;8(7):e67873. doi: 10.1371/journal.pone.0067873. Print 2013. PLoS One. 2013. PMID: 23874461 Free PMC article.
-
DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway.Blood. 2011 Jun 16;117(24):6589-99. doi: 10.1182/blood-2011-01-331363. Epub 2011 May 2. Blood. 2011. PMID: 21536857
-
The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways.Blood. 2008 Aug 15;112(4):1299-307. doi: 10.1182/blood-2008-01-136473. Epub 2008 Jun 9. Blood. 2008. PMID: 18541725 Free PMC article.
-
Cell-to-Cell Spread of HIV and Viral Pathogenesis.Adv Virus Res. 2016;95:43-85. doi: 10.1016/bs.aivir.2016.03.001. Epub 2016 Apr 4. Adv Virus Res. 2016. PMID: 27112280 Review.
-
Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2.Immunobiology. 2004;209(1-2):179-90. doi: 10.1016/j.imbio.2004.03.004. Immunobiology. 2004. PMID: 15481152 Review.
Cited by
-
HIV-1 Trans Infection of CD4(+) T Cells by Professional Antigen Presenting Cells.Scientifica (Cairo). 2013;2013:164203. doi: 10.1155/2013/164203. Epub 2013 May 7. Scientifica (Cairo). 2013. PMID: 24278768 Free PMC article. Review.
-
Iron status in HIV-1 infection: implications in disease pathology.BMC Clin Pathol. 2012 Dec 17;12:26. doi: 10.1186/1472-6890-12-26. BMC Clin Pathol. 2012. PMID: 23245266 Free PMC article.
-
C-Type Lectin Receptors in Antiviral Immunity and Viral Escape.Front Immunol. 2018 Mar 26;9:590. doi: 10.3389/fimmu.2018.00590. eCollection 2018. Front Immunol. 2018. PMID: 29632536 Free PMC article. Review.
-
Molecular control of steady-state dendritic cell maturation and immune homeostasis.Annu Rev Immunol. 2013;31:743-91. doi: 10.1146/annurev-immunol-020711-074929. Epub 2013 Jan 17. Annu Rev Immunol. 2013. PMID: 23330953 Free PMC article. Review.
-
Dendritic cell immunoreceptor is a new target for anti-AIDS drug development: identification of DCIR/HIV-1 inhibitors.PLoS One. 2013 Jul 9;8(7):e67873. doi: 10.1371/journal.pone.0067873. Print 2013. PLoS One. 2013. PMID: 23874461 Free PMC article.
References
-
- Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 1999;163:1973–1983. - PubMed
-
- Fujikado N, Saijo S, Yonezawa T, Shimamori K, Ishii A, et al. DCIR deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–180. - PubMed
-
- Eklow C, Makrygiannakis D, Backdahl L, Padyukov L, Ulfgren AK, et al. Cellular distribution of the C-type II lectin dendritic cell immunoreceptor (DCIR) and its expression in the rheumatic joint: identification of a subpopulation of DCIR+ T cells. Ann Rheum Dis. 2008;67:1742–1749. - PubMed
-
- Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

