Amyloid-β oligomer specificity mediated by the IgM isotype--implications for a specific protective mechanism exerted by endogenous auto-antibodies
- PMID: 21085663
- PMCID: PMC2978096
- DOI: 10.1371/journal.pone.0013928
Amyloid-β oligomer specificity mediated by the IgM isotype--implications for a specific protective mechanism exerted by endogenous auto-antibodies
Abstract
Background: Alzheimers disease (AD) has been strongly linked to an anomalous self-assembly of the amyloid-β peptide (Aβ). The correlation between clinical symptoms of AD and Aβ depositions is, however, weak. Instead small and soluble Aβ oligomers are suggested to exert the major pathological effects. In strong support of this notion, immunological targeting of Aβ oligomers in AD mice-models shows that memory impairments can be restored without affecting the total burden of Aβ deposits. Consequently a specific immunological targeting of Aβ oligomers is of high therapeutic interest.
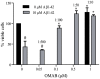
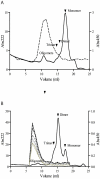
Methodology/principal findings: Previously the generation of conformational-dependent oligomer specific anti-Aβ antibodies has been described. However, to avoid the difficult task of identifying a molecular architecture only present on oligomers, we have focused on a more general approach based on the hypothesis that all oligomers expose multiple identical epitopes and therefore would have an increased binding to a multivalent receptor. Using the polyvalent IgM immunoglobulin we have developed a monoclonal anti-Aβ antibody (OMAB). OMAB only demonstrates a weak interaction with Aβ monomers and dimers having fast on and off-rate kinetics. However, as an effect of avidity, its interaction with Aβ-oligomers results in a strong complex with an exceptionally slow off-rate. Through this mechanism a selectivity towards Aβ oligomers is acquired and OMAB fully inhibits the cytotoxic effect exerted by Aβ(1-42) at highly substoichiometric ratios. Anti-Aβ auto-antibodies of IgM isotype are frequently present in the sera of humans. Through a screen of endogenous anti-Aβ IgM auto-antibodies from a group of healthy individuals we show that all displays a preference for oligomeric Aβ.
Conclusions/significance: Taken together we provide a simple and general mechanism for targeting of oligomers without the requirement of conformational-dependent epitopes. In addition, our results suggest that IgM anti-Aβ auto-antibodies may exert a more specific protective mechanism in vivo than previously anticipated.
Conflict of interest statement
Figures



References
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, et al. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994;13:45–53. - PubMed
-
- Katzman R. Differential diagnosis of dementing illnesses. Neurol Clin. 1986;4:329–340. - PubMed
-
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. - PubMed
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. - PubMed
-
- Younkin SG. Amyloid beta vaccination: reduced plaques and improved cognition. Nat Med. 2001;7:18–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

