Functional analysis of Ficolin-3 mediated complement activation
- PMID: 21085669
- PMCID: PMC2978102
- DOI: 10.1371/journal.pone.0015443
Functional analysis of Ficolin-3 mediated complement activation
Abstract
The recognition molecules of the lectin complement pathway are mannose-binding lectin and Ficolin -1, -2 and -3. Recently deficiency of Ficolin-3 was found to be associated with life threatening infections. Thus, we aimed to develop a functional method based on the ELISA platform for evaluating Ficolin-3 mediated complement activation that could be applicable for research and clinical use. Bovine serum albumin (BSA) was acetylated (acBSA) and chosen as a solid phase ligand for Ficolins in microtiter wells. Binding of Ficolins on acBSA was evaluated, as was functional complement activation assessed by C4, C3 and terminal complement complex (TCC) deposition. Serum Ficolin-3 bound to acBSA in a calcium dependent manner, while only minimal binding of Ficolin-2 and no binding of Ficolin-1 were observed. No binding to normal BSA was seen for any of the Ficolins. Serum C4, C3 and TCC deposition on acBSA were dependent only on Ficolin-3 in appropriate serum dilutions. Deposition of down stream complement components correlated highly significantly with the serum concentration of Ficolin-3 but not with Ficolin-2 in healthy donors. To make the assay robust for clinical use a chemical compound was applied to the samples that inhibited interference from the classical pathway due to the presence of anti-BSA antibodies in some sera. We describe a novel functional method for measuring complement activation mediated by Ficolin-3 in human serum up to the formation of TCC. The assay provides the possibility to diagnose functional and genetic defects of Ficolin-3 and down stream components in the lectin complement pathway.
Conflict of interest statement
Figures

 ), rFicolin-2 (
), rFicolin-2 ( ), rFicolin-3 (
), rFicolin-3 ( ) at (A) 30 min at 37°C or (B) 3 h at RT. Binding was detected with monoclonal antibodies directed against the individual proteins. Graphs show mean ± SD (n = 4).
) at (A) 30 min at 37°C or (B) 3 h at RT. Binding was detected with monoclonal antibodies directed against the individual proteins. Graphs show mean ± SD (n = 4).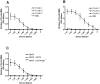
 ), Ficolin-2 (
), Ficolin-2 ( ), Ficolin-3 (
), Ficolin-3 ( ) or MBL (
) or MBL ( ) was subsequently detected with monoclonal antibodies directed against the individual proteins. (C) acBSA incubated with: NHSP (
) was subsequently detected with monoclonal antibodies directed against the individual proteins. (C) acBSA incubated with: NHSP ( ), NHSP with 10 mM EDTA (
), NHSP with 10 mM EDTA ( ) or NHSP with 10 mM EGTA and 5 mM Mg2+ (
) or NHSP with 10 mM EGTA and 5 mM Mg2+ ( ). Binding was detected with a monoclonal antibody against Ficolin-3. All graphs show mean ± SD (n = 3).
). Binding was detected with a monoclonal antibody against Ficolin-3. All graphs show mean ± SD (n = 3).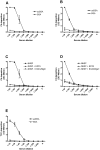
 ) or BSA (
) or BSA ( ) was detected using a polyclonal antibody. (C–D) Microtiter wells were coated with acBSA and subsequently incubated with NHSP (
) was detected using a polyclonal antibody. (C–D) Microtiter wells were coated with acBSA and subsequently incubated with NHSP ( ), NHSP with 10 mM EDTA (
), NHSP with 10 mM EDTA ( ) or NHSP with 10 mM EGTA and 5 mM Mg2+ (
) or NHSP with 10 mM EGTA and 5 mM Mg2+ ( ) for 30 min at 37°C. (C) C4 or (D) C3 deposition was detected as described above. (E) Finally, microtiter wells were coated with acBSA (
) for 30 min at 37°C. (C) C4 or (D) C3 deposition was detected as described above. (E) Finally, microtiter wells were coated with acBSA ( ) or BSA (
) or BSA ( ) and subsequently incubated with NHSP in serial dilution for 45 min at 37°C and TCC-deposition on acBSA was detected using a monoclonal antibody. All graphs show mean ± SD (n = 4).
) and subsequently incubated with NHSP in serial dilution for 45 min at 37°C and TCC-deposition on acBSA was detected using a monoclonal antibody. All graphs show mean ± SD (n = 4).
 ), Ficolin-2 depleted serum (-♦-), Ficolin-2 depleted serum with addition of 5 µg/ml rFicolin-2 (
), Ficolin-2 depleted serum (-♦-), Ficolin-2 depleted serum with addition of 5 µg/ml rFicolin-2 ( ). (C) Shows TCC deposition for: NHSP (
). (C) Shows TCC deposition for: NHSP ( ), Ficolin-3 depleted serum (-▪-) and Ficolin-3 depleted serum with addition of 25 µg/ml rFicolin-3 (
), Ficolin-3 depleted serum (-▪-) and Ficolin-3 depleted serum with addition of 25 µg/ml rFicolin-3 ( ). (D) Shows TCC deposition for: NHSP (
). (D) Shows TCC deposition for: NHSP ( ), Ficolin-3 deficient serum −/− (-▴-), Ficolin-3 deficient serum −/− with addition of 25 µg/ml rFicolin-3 (
), Ficolin-3 deficient serum −/− (-▴-), Ficolin-3 deficient serum −/− with addition of 25 µg/ml rFicolin-3 ( ). All graphs show mean ± SD of duplicate wells.
). All graphs show mean ± SD of duplicate wells.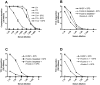
 ), C3 (
), C3 ( ) or TCC (
) or TCC ( ); and in the presence of SPS: C4 (
); and in the presence of SPS: C4 ( ), C3 (-•-) or TCC (-□-). (B–D) Different sera pre-incubated on ice with SPS and then incubated for 45 min at 37°C in microtiter plates coated with acBSA before TCC deposition was detected with a monoclonal antibody. (B) Shows TCC deposition in the presence of SPS for: NHSP (
), C3 (-•-) or TCC (-□-). (B–D) Different sera pre-incubated on ice with SPS and then incubated for 45 min at 37°C in microtiter plates coated with acBSA before TCC deposition was detected with a monoclonal antibody. (B) Shows TCC deposition in the presence of SPS for: NHSP ( ), Ficolin-2 depleted serum (- ♦ -), Ficolin-2 depleted serum with addition of 5 µg/ml rFicolin-2 (
), Ficolin-2 depleted serum (- ♦ -), Ficolin-2 depleted serum with addition of 5 µg/ml rFicolin-2 ( ). (C) Shows TCC deposition in the presence of SPS for: NHSP (
). (C) Shows TCC deposition in the presence of SPS for: NHSP ( ), Ficolin-3 depleted serum (-▪-) and Ficolin-3 depleted serum with addition of 25 µg/ml rFicolin-3 (
), Ficolin-3 depleted serum (-▪-) and Ficolin-3 depleted serum with addition of 25 µg/ml rFicolin-3 ( ). (D) Shows TCC deposition in the presence of SPS for: NHSP (-▪-), Ficolin-3 deficient serum −/− (-▴-), Ficolin-3 deficient serum −/− with addition of 25 µg/ml rFicolin-3 (
). (D) Shows TCC deposition in the presence of SPS for: NHSP (-▪-), Ficolin-3 deficient serum −/− (-▴-), Ficolin-3 deficient serum −/− with addition of 25 µg/ml rFicolin-3 ( ). All graphs show mean ± SD of duplicate wells.
). All graphs show mean ± SD of duplicate wells.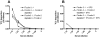
 ), Ficolin-3 deficient serum −/− depleted of Ficolin-2 (
), Ficolin-3 deficient serum −/− depleted of Ficolin-2 ( ), Ficolin-3 deficient serum −/− depleted of Ficolin-2 with addition of 5 µg/ml rFicolin-2 (
), Ficolin-3 deficient serum −/− depleted of Ficolin-2 with addition of 5 µg/ml rFicolin-2 ( ). TCC deposition was detected with a monoclonal antibody. Graphs show mean ± SD of duplicate wells.
). TCC deposition was detected with a monoclonal antibody. Graphs show mean ± SD of duplicate wells.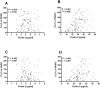
 ), C2 deficient serum (
), C2 deficient serum ( ), C5 deficient serum (
), C5 deficient serum ( ) or properdin deficient serum (
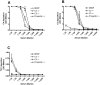
) or properdin deficient serum ( ) in serial dilutions for 30 min at 37°C. (A) C4 deposition and (B) C3 deposition on acBSA measured using polyclonal antibodies. (C) TCC deposition on acBSA measured using a monoclonal antibody. All graphs show mean ± SD (n = 3).
) in serial dilutions for 30 min at 37°C. (A) C4 deposition and (B) C3 deposition on acBSA measured using polyclonal antibodies. (C) TCC deposition on acBSA measured using a monoclonal antibody. All graphs show mean ± SD (n = 3).Similar articles
-
Ficolin-2 reveals different analytical and biological properties dependent on different sample handling procedures.Mol Immunol. 2013 Dec;56(4):406-12. doi: 10.1016/j.molimm.2013.05.233. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911396
-
Cholesterol Crystals Activate the Lectin Complement Pathway via Ficolin-2 and Mannose-Binding Lectin: Implications for the Progression of Atherosclerosis.J Immunol. 2016 Jun 15;196(12):5064-74. doi: 10.4049/jimmunol.1502595. Epub 2016 May 16. J Immunol. 2016. PMID: 27183610
-
Ficolins and the lectin pathway of complement in patients with systemic lupus erythematosus.Mol Immunol. 2015 Feb;63(2):209-14. doi: 10.1016/j.molimm.2014.07.003. Epub 2014 Jul 25. Mol Immunol. 2015. PMID: 25069872
-
MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement.Mol Immunol. 2009 Sep;46(14):2737-44. doi: 10.1016/j.molimm.2009.05.005. Epub 2009 Jun 7. Mol Immunol. 2009. PMID: 19501910 Review.
-
Structural and functional overview of the lectin complement pathway: its molecular basis and physiological implication.Arch Immunol Ther Exp (Warsz). 2013 Aug;61(4):273-83. doi: 10.1007/s00005-013-0229-y. Epub 2013 Apr 7. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23563865 Review.
Cited by
-
Integrative genetic and immune cell analysis of plasma proteins in healthy donors identifies novel associations involving primary immune deficiency genes.Genome Med. 2022 Mar 9;14(1):28. doi: 10.1186/s13073-022-01032-y. Genome Med. 2022. PMID: 35264221 Free PMC article.
-
Ficolins do not alter host immune responses to lipopolysaccharide-induced inflammation in vivo.Sci Rep. 2017 Jun 20;7(1):3852. doi: 10.1038/s41598-017-04121-w. Sci Rep. 2017. PMID: 28634324 Free PMC article.
-
A Metalloproteinase Mirolysin of Tannerella forsythia Inhibits All Pathways of the Complement System.J Immunol. 2015 Sep 1;195(5):2231-40. doi: 10.4049/jimmunol.1402892. Epub 2015 Jul 24. J Immunol. 2015. PMID: 26209620 Free PMC article.
-
The C-type lectin of the aggrecan G3 domain activates complement.PLoS One. 2013 Apr 15;8(4):e61407. doi: 10.1371/journal.pone.0061407. Print 2013. PLoS One. 2013. PMID: 23596522 Free PMC article.
-
Seroconversion stages COVID19 into distinct pathophysiological states.medRxiv [Preprint]. 2020 Dec 7:2020.12.05.20244442. doi: 10.1101/2020.12.05.20244442. medRxiv. 2020. Update in: Elife. 2021 Mar 16;10:e65508. doi: 10.7554/eLife.65508. PMID: 33330890 Free PMC article. Updated. Preprint.
References
-
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. - PubMed
-
- Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. - PubMed
-
- Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. - PubMed
-
- Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–3888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

