Strategies for increasing recruitment to randomised controlled trials: systematic review
- PMID: 21085696
- PMCID: PMC2976724
- DOI: 10.1371/journal.pmed.1000368
Strategies for increasing recruitment to randomised controlled trials: systematic review
Abstract
Background: Recruitment of participants into randomised controlled trials (RCTs) is critical for successful trial conduct. Although there have been two previous systematic reviews on related topics, the results (which identified specific interventions) were inconclusive and not generalizable. The aim of our study was to evaluate the relative effectiveness of recruitment strategies for participation in RCTs.
Methods and findings: A systematic review, using the PRISMA guideline for reporting of systematic reviews, that compared methods of recruiting individual study participants into an actual or mock RCT were included. We searched MEDLINE, Embase, The Cochrane Library, and reference lists of relevant studies. From over 16,000 titles or abstracts reviewed, 396 papers were retrieved and 37 studies were included, in which 18,812 of at least 59,354 people approached agreed to participate in a clinical RCT. Recruitment strategies were broadly divided into four groups: novel trial designs (eight studies), recruiter differences (eight studies), incentives (two studies), and provision of trial information (19 studies). Strategies that increased people's awareness of the health problem being studied (e.g., an interactive computer program [relative risk (RR) 1.48, 95% confidence interval (CI) 1.00-2.18], attendance at an education session [RR 1.14, 95% CI 1.01-1.28], addition of a health questionnaire [RR 1.37, 95% CI 1.14-1.66]), or a video about the health condition (RR 1.75, 95% CI 1.11-2.74), and also monetary incentives (RR1.39, 95% CI 1.13-1.64 to RR 1.53, 95% CI 1.28-1.84) improved recruitment. Increasing patients' understanding of the trial process, recruiter differences, and various methods of randomisation and consent design did not show a difference in recruitment. Consent rates were also higher for nonblinded trial design, but differential loss to follow up between groups may jeopardise the study findings. The study's main limitation was the necessity of modifying the search strategy with subsequent search updates because of changes in MEDLINE definitions. The abstracts of previous versions of this systematic review were published in 2002 and 2007.
Conclusion: Recruitment strategies that focus on increasing potential participants' awareness of the health problem being studied, its potential impact on their health, and their engagement in the learning process appeared to increase recruitment to clinical studies. Further trials of recruitment strategies that target engaging participants to increase their awareness of the health problems being studied and the potential impact on their health may confirm this hypothesis. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
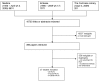

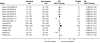
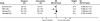


References
-
- Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–352. - PubMed
-
- Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995;87:1747–1759. - PubMed
-
- Walson PD. Patient recruitment: US perspective. Pediatrics. 1999;104:619–622. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

