Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection
- PMID: 21085698
- PMCID: PMC2976761
- DOI: 10.1371/journal.pone.0015398
Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection
Abstract
Background: Campylobacter jejuni is the most prevalent cause of bacterial gastroenteritis worldwide. Despite the significant health burden this infection presents, molecular understanding of C. jejuni-mediated disease pathogenesis remains poorly defined. Here, we report the characterisation of the early, innate immune response to C. jejuni using an ex-vivo human gut model of infection. Secondly, impact of bacterial-driven dendritic cell activation on T-cell mediated immunity was also sought.
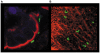
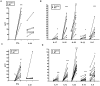
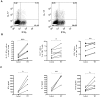
Methodology: Healthy, control paediatric terminal ileum or colonic biopsy tissue was infected with C. jejuni for 8-12 hours. Bacterial colonisation was followed by confocal microscopy and mucosal innate immune responses measured by ELISA. Marked induction of IFNγ with modest increase in IL-22 and IL-17A was noted. Increased mucosal IL-12, IL-23, IL-1β and IL-6 were indicative of a cytokine milieu that may modulate subsequent T-cell mediated immunity. C. jejuni-driven human monocyte-derived dendritic cell activation was followed by analyses of T cell immune responses utilising flow cytometry and ELISA. Significant increase in Th-17, Th-1 and Th-17/Th-1 double-positive cells and corresponding cytokines was observed. The ability of IFNγ, IL-22 and IL-17 cytokines to exert host defence via modulation of C. jejuni adhesion and invasion to intestinal epithelia was measured by standard gentamicin protection assay.
Conclusions: Both innate and adaptive T cell-immunity to C. jejuni infection led to the release of IFNγ, IL-22 and IL-17A; suggesting a critical role for this cytokine triad in establishing host anti-microbial immunity during the acute and effectors phase of infection. In addition, to their known anti-microbial functions; IL-17A and IL-17F reduced the number of intracellular C. jejuni in intestinal epithelia, highlighting a novel aspect of how IL-17 family members may contribute to protective immunity against C. jejuni.
Conflict of interest statement
Figures
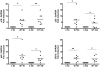

References
-
- Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. - PubMed
-
- Poly F, Guerry P. Pathogenesis of Campylobacter. Curr Opin Gastroenterol. 2008;24:27–31. - PubMed
-
- Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. - PubMed
-
- Vucic S, Kiernan MC, Cornblath DR. Guillain-Barre syndrome: An update. J Clin Neurosci. 2009;16:733–741. - PubMed
-
- Oberhelman RA, Gilman RH, Sheen P, Cordova J, Taylor DN, et al. Campylobacter transmission in a Peruvian shantytown: a longitudinal study using strain typing of campylobacter isolates from chickens and humans in household clusters. J Infect Dis. 2003;187:260–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

