Rates of intercalator-driven platination of DNA determined by a restriction enzyme cleavage inhibition assay
- PMID: 21086002
- PMCID: PMC3049956
- DOI: 10.1007/s00775-010-0733-z
Rates of intercalator-driven platination of DNA determined by a restriction enzyme cleavage inhibition assay
Abstract
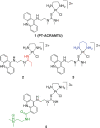
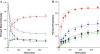
A restriction enzyme cleavage inhibition assay was designed to determine the rates of DNA platination by four non-cross-linking platinum-acridine agents represented by the formula [Pt(am(2))LCl](NO(3))(2), where am is a diamine nonleaving group and L is an acridine derived from the intercalator 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea (ACRAMTU). The formation of monofunctional adducts in the target sequence 5'-CGA was studied in a 40-base-pair probe containing the EcoRI restriction site GAATTC. The time dependence of endonuclease inhibition was quantitatively analyzed by polyacrylamide gel electrophoresis. The formation of monoadducts is approximately 3 times faster with double-stranded DNA than with simple nucleic acid fragments. Compound 1 (am(2) is ethane-1,2-diamine, L is ACRAMTU) reacts with a first-order rate constant of k (obs) = 1.4 ± 0.37 × 10(-4) s(-1) (t (1/2) = 83 ± 22 min). Replacement of the thiourea group in ACRAMTU with an amidine group (compound 2) accelerates the rate by fourfold (k (obs) = 5.7 ± 0.58 × 10(-4) s(-1), t (1/2) = 21 ± 2 min), and introduction of a propane-1,3-diamine nonleaving group results in a 1.5-fold enhancement in reactivity (compound 3, k (obs) = 2.1 ± 0.40 × 10(-4) s(-1), t (1/2) = 55 ± 10 min) compared with the prototype. Derivative 4, containing a 4,9-disubstituted acridine threading intercalator, was the least reactive compound in the series (k (obs) = 1.1 ± 0.40 × 10(-4) s(-1), t (1/2) = 104 ± 38 min). The data suggest a correlation may exist between the binding rates and the biological activity of the compounds. Potential pharmacological advantages of rapid formation of cytotoxic monofunctional adducts over the common purine-purine cross-links are discussed.
Figures


References
-
- Kelland L. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Anticancer Agents Med Chem. 2007;7:3–18. - PubMed
-
- van de Vaart PJ, Belderbos J, de Jong D, Sneeuw KC, Majoor D, Bartelink H, Begg AC. Int J Cancer. 2000;89:160–166. - PubMed
-
- Fujii T, Toyooka S, Ichimura K, Fujiwara Y, Hotta K, Soh J, Suehisa H, Kobayashi N, Aoe M, Yoshino T, Kiura K, Date H. Lung Cancer. 2008;59:377–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

