In vivo activity of novel anti-ErbB2 antibody chA21 alone and with Paclitaxel or Trastuzumab in breast and ovarian cancer xenograft models
- PMID: 21086124
- PMCID: PMC11029528
- DOI: 10.1007/s00262-010-0937-7
In vivo activity of novel anti-ErbB2 antibody chA21 alone and with Paclitaxel or Trastuzumab in breast and ovarian cancer xenograft models
Abstract
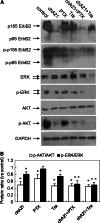
It was well studied that ErbB2 (HER2/p185(her2/neu)) overexpression in human malignant cancers correlates with poor prognosis and chemo-resistance. Although Trastuzumab (Herceptin) has been widely used in patients with ErbB2-overexpressing metastatic breast cancer, many patients either do not respond to Trastuzumab therapy or progress within 1 year of initiating Trastuzumab treatment. Previously, we reported a novel tumor-inhibitory antibody chA21, which recognized ErbB2 extracellular domain with an epitope distinct from other tumor-inhibitory anti-ErbB2 antibodies. Here, we report that chA21 combined with Paclitaxel or Trastuzumab significantly enhances the tumor-inhibition effects on ErbB2-overexpressing breast and ovarian cancer in xenograft mice. Moreover, the study reveals that the effects by chA21 to cause an enhanced inhibition on cancer cell proliferation and angiogenesis was highly associated with the intrinsic ability of chA21 to down-regulate ErbB2 receptor, inhibit downstream MAPK and PI3K-AKT signal transduction and activate natural killer cells. Our findings show that chA21 may represent a unique anti-ErbB2 antibody with potentials as therapeutic candidate alone or combination with other anti-ErbB2 reagents in cancer therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Therapeutic targeting of erbB3 with MM-121/SAR256212 enhances antitumor activity of paclitaxel against erbB2-overexpressing breast cancer.Breast Cancer Res. 2013;15(5):R101. doi: 10.1186/bcr3563. Breast Cancer Res. 2013. PMID: 24168763 Free PMC article.
-
Anticancer activity of Celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers.Cancer Biol Ther. 2011 Jan 15;11(2):263-76. doi: 10.4161/cbt.11.2.13959. Epub 2011 Jan 15. Cancer Biol Ther. 2011. PMID: 21088503 Free PMC article.
-
Enhanced sensitization to taxol-induced apoptosis by herceptin pretreatment in ErbB2-overexpressing breast cancer cells.Cancer Res. 2002 Oct 15;62(20):5703-10. Cancer Res. 2002. PMID: 12384528
-
Mechanisms of ErbB2-mediated paclitaxel resistance and trastuzumab-mediated paclitaxel sensitization in ErbB2-overexpressing breast cancers.Semin Oncol. 2001 Oct;28(5 Suppl 16):12-7. doi: 10.1016/s0093-7754(01)90277-5. Semin Oncol. 2001. PMID: 11706391 Review.
-
The ErbB2 signaling network as a target for breast cancer therapy.J Mammary Gland Biol Neoplasia. 2006 Jan;11(1):13-25. doi: 10.1007/s10911-006-9009-1. J Mammary Gland Biol Neoplasia. 2006. PMID: 16947083 Review.
Cited by
-
Analysis of microarray-identified genes and microRNAs associated with drug resistance in ovarian cancer.Int J Clin Exp Pathol. 2015 Jun 1;8(6):6847-58. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26261572 Free PMC article.
-
Development and Characterization of a Humanized Anti-HER2 Antibody HuA21 with Potent Anti-Tumor Properties in Breast Cancer Cells.Int J Mol Sci. 2016 Apr 15;17(4):563. doi: 10.3390/ijms17040563. Int J Mol Sci. 2016. PMID: 27092488 Free PMC article.
-
In vivo hyperspectral imaging of microvessel response to trastuzumab treatment in breast cancer xenografts.Biomed Opt Express. 2014 Jun 16;5(7):2247-61. doi: 10.1364/BOE.5.002247. eCollection 2014 Jul 1. Biomed Opt Express. 2014. PMID: 25071962 Free PMC article.
-
MiR-1268b confers chemosensitivity in breast cancer by targeting ERBB2-mediated PI3K-AKT pathway.Oncotarget. 2017 Aug 9;8(52):89631-89642. doi: 10.18632/oncotarget.20099. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163776 Free PMC article.
-
Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity-From Bench to Bedside.Biomedicines. 2020 Dec 7;8(12):577. doi: 10.3390/biomedicines8120577. Biomedicines. 2020. PMID: 33297351 Free PMC article.
References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

