Facile trypsin immobilization in polymeric membranes for rapid, efficient protein digestion
- PMID: 21087034
- PMCID: PMC3052767
- DOI: 10.1021/ac101857j
Facile trypsin immobilization in polymeric membranes for rapid, efficient protein digestion
Abstract
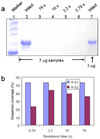
Sequential adsorption of poly(styrene sulfonate) and trypsin in nylon membranes provides a simple, inexpensive method to create stable, microporous reactors for fast protein digestion. The high local trypsin concentration and short radial diffusion distances in membrane pores facilitate proteolysis in residence times of a few seconds, and the minimal pressure drop across the thin membranes allows their use in syringe filters. Membrane digestion and subsequent MS analysis of bovine serum albumin provide 84% sequence coverage, which is higher than the 71% coverage obtained with in-solution digestion for 16 h or the <50% sequence coverages of other methods that employ immobilized trypsin. Moreover, trypsin-modified membranes digest protein in the presence of 0.05 wt % sodium dodecyl sulfate (SDS), whereas in-solution digestion under similar conditions yields no peptide signals in mass spectra even after removal of SDS. These membrane reactors, which can be easily prepared in any laboratory, have a shelf life of several months and continuously digest protein for at least 33 h without significant loss of activity.
Figures





Similar articles
-
Trypsin immobilization in ordered porous polymer membranes for effective protein digestion.Anal Chim Acta. 2016 Feb 4;906:156-164. doi: 10.1016/j.aca.2015.11.042. Epub 2015 Dec 17. Anal Chim Acta. 2016. PMID: 26772135
-
Preparation and characterization of a packed bead immobilized trypsin reactor integrated into a PDMS microfluidic chip for rapid protein digestion.Talanta. 2017 May 1;166:275-283. doi: 10.1016/j.talanta.2017.01.060. Epub 2017 Jan 25. Talanta. 2017. PMID: 28213235
-
A hydrophilic immobilized trypsin reactor with N-vinyl-2-pyrrolidinone modified polymer microparticles as matrix for highly efficient protein digestion with low peptide residue.J Chromatogr A. 2012 Jul 13;1246:111-6. doi: 10.1016/j.chroma.2012.03.014. Epub 2012 Mar 9. J Chromatogr A. 2012. PMID: 22446077
-
Enzyme-immobilized reactors for rapid and efficient sample preparation in MS-based proteomic studies.Proteomics. 2013 Feb;13(3-4):457-66. doi: 10.1002/pmic.201200272. Epub 2013 Jan 14. Proteomics. 2013. PMID: 23255229 Review.
-
Functionalizing Microporous Membranes for Protein Purification and Protein Digestion.Annu Rev Anal Chem (Palo Alto Calif). 2015;8:81-100. doi: 10.1146/annurev-anchem-071114-040255. Epub 2015 May 18. Annu Rev Anal Chem (Palo Alto Calif). 2015. PMID: 26001953 Review.
Cited by
-
Systematic Evaluation of Immobilized Trypsin-Based Fast Protein Digestion for Deep and High-Throughput Bottom-Up Proteomics.Proteomics. 2018 May;18(9):e1700432. doi: 10.1002/pmic.201700432. Epub 2018 Apr 15. Proteomics. 2018. PMID: 29577644 Free PMC article.
-
Study of the geometry of open channels in a layer-bed-type microfluidic immobilized enzyme reactor.Anal Bioanal Chem. 2021 Oct;413(25):6321-6332. doi: 10.1007/s00216-021-03588-x. Epub 2021 Aug 10. Anal Bioanal Chem. 2021. PMID: 34378068 Free PMC article.
-
A colorimetric method for monitoring tryptic digestion prior to shotgun proteomics.Int J Proteomics. 2014;2014:125482. doi: 10.1155/2014/125482. Epub 2014 Feb 10. Int J Proteomics. 2014. PMID: 24678421 Free PMC article.
-
Reagent-Free Immobilization of Industrial Lipases to Develop Lipolytic Membranes with Self-Cleaning Surfaces.Membranes (Basel). 2022 Jun 9;12(6):599. doi: 10.3390/membranes12060599. Membranes (Basel). 2022. PMID: 35736306 Free PMC article.
-
Development of an In-Line Enzyme Reactor Integrated into a Capillary Electrophoresis System.Molecules. 2021 Sep 29;26(19):5902. doi: 10.3390/molecules26195902. Molecules. 2021. PMID: 34641446 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

