Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy
- PMID: 21087247
- PMCID: PMC3058802
- DOI: 10.1111/j.1528-1167.2010.02789.x
Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy
Abstract
Purpose: Study aims were (1) to document and examine associations between parent-report and electronic monitoring (EM) of pediatric antiepileptic drug (AED) adherence, (2) to determine the sensitivity and specificity of parent-reported adherence, and (3) to develop a correction factor for parent-reported adherence.
Methods: Participants included 111 consecutive children with new-onset epilepsy (M(age) = 7.2 ± 2.0; 61.3% male; 75.8% Caucasian) and their primary caregivers. AED adherence was electronically monitored for 3 months prior to the 4-month clinic follow-up visit. Parent-reported adherence captured adherence 1-week prior to the clinic visit. For specificity/sensitivity analyses of parent-reported adherence, cut points of 50%, 80%, and 90% were used with electronically monitored adherence calculated 1-week prior to the clinic visit as the reference criterion.
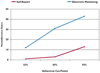
Key findings: Electronically monitored adherence (80.3%) was significantly lower than parent-reported adherence (96.5%; p < 0.0001) 1-week prior to the clinic visit, but both were significantly correlated (rho = 0.46, p < 0.001). The 90% parent-reported adherence cut point demonstrated the most sensitivity and specificity to electronically monitored adherence; however, specificity was still only 28%. A correction factor of 0.83 was identified as a reliable adjustment for parent-reported adherence when compared to electronically monitored adherence.
Significance: Although EM is the gold standard of adherence measurement for pediatric epilepsy, it is often not clinically feasible to integrate it into routine clinical care. Therefore, use of a correction factor for interpreting parent-reported adherence holds promise as a reliable clinical tool. With reliable adherence measurement, clinicians can provide adherence interventions with the hope of optimizing health outcomes for children with epilepsy.
Wiley Periodicals, Inc. © 2010 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures
References
-
- Al-Faris EA, Abdulghani HM, Mahdi AH, Salih MA, Al-Kordi AG. Compliance with appointments and medications in a pediatric neurology clinic at a university hospital in Riyadh, Saudi Arabia. Saudi Med J. 2002;23:969–974. - PubMed
-
- Asadi-Pooya AA. Drug compliance of children and adolescents with epilepsy. Seizure. 2005;14:393–395. - PubMed
-
- Asato MR, Manjunath R, Sheth RD, Phelps SJ, Wheless JW, Hovinga CA, Pina-Garza JE, Haskins LS, Zingaro WM. Adolescent and caregiver experiences with epilepsy. J Child Neurol. 2009;24:562–571. - PubMed
-
- Bassili A, Omar T, Zaki A, Abdel-Fattah M, Tognoni G. Pattern of diagnostic and therapeutic care of childhood epilepsy in Alexandria, Egypt. Int J Qual Health Care. 2002;14:277–284. - PubMed
-
- Berg JS, Dischler J, Wagner DJ, Raia JJ, Palmer-Shevlin N. Medication compliance: A healthcare problem. Ann Pharmacother. 1993;27(9 Suppl):S1–S24. - PubMed