Acute pancreatitis with organ dysfunction associates with abnormal blood lymphocyte signaling: controlled laboratory study
- PMID: 21087472
- PMCID: PMC3220021
- DOI: 10.1186/cc9329
Acute pancreatitis with organ dysfunction associates with abnormal blood lymphocyte signaling: controlled laboratory study
Abstract
Introduction: Severe acute pancreatitis is associated with systemic inflammation, compensatory immune suppression, secondary infections, vital organ dysfunction, and death.Our study purpose was to delineate signaling profiles of circulating lymphocytes in acute pancreatitis complicated by organ dysfunction.
Methods: Sixteen patients with acute pancreatitis, dysfunction of vital organ(s), and immune suppression (proportion of HLA-DR Human Leukocyte Antigen - DR - positive monocytes < 80%) participated. Healthy volunteers served as reference subjects. Using phospho-specific whole blood flow cytometry we studied lymphocyte phosphorylation of nuclear factor-κB (NFκB), mitogen-activated protein kinases p38 and extracellular signal-regulated kinases (ERK)1/2, and signal transducers and activators of transcription (STATs) 1, 3, and 6. Statistical comparisons were performed with the Wilcoxon-Mann-Whitney test.
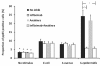
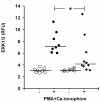
Results: In blood samples supplemented with tumor necrosis factor, E. coli or S. aureus, phosphorylation levels of NFκB were lower and levels of p38 were higher in patients with acute pancreatitis than healthy subjects. Low NFκB activation involved CD3+CD4+ and CD3+CD8+ lymphocytes. ERK1/2 phosphorylation induced by co-stimulation with phorbol 12-myristate 13-acetate and calcium ionophore A23187 was depressed in patients. STAT3 was constitutively activated in patients' CD3+CD4+ and CD3+CD8+ lymphocytes. Also, IL-6-induced STAT1 phosphorylation was impaired while IL-4-induced STAT6 phosphorylation was enhanced.
Conclusions: Lymphocytes of patients with acute pancreatitis, organ dysfunction and immune suppression show impaired NFκB activation, which increases infection risk and enhanced p38 activation, which sustains inflammation. Secondly, they indicate constitutive STAT3 activation, which may favor Th17 lineage of CD4+ lymphocyte differentiation. Thirdly, they reveal impaired STAT1 activation and enhanced STAT6 activation, denoting a shift from Th1 towards Th2 differentiation.
Figures



Comment in
-
The first step in utilizing immune-modulating therapies: immune status determination.Crit Care. 2011;15(1):108. doi: 10.1186/cc9397. Epub 2011 Jan 24. Crit Care. 2011. PMID: 21349138 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

