Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk
- PMID: 21087507
- PMCID: PMC2998478
- DOI: 10.1186/1476-4598-9-295
Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk
Abstract
Background: Adult human mesenchymal stem cells (hMSC) have been shown to home to sites of carcinoma and affect biological processes, including tumour growth and metastasis. Previous findings have been conflicting and a clear understanding of the effects of hMSCs on cancer remains to be established. Therefore, we set out to investigate the impact of hMSCs on the oestrogen receptor positive, hormone-dependent breast carcinoma cell line MCF-7.
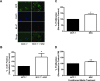
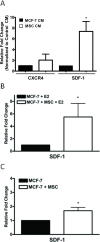
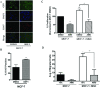
Results: In this study, we show the effects of hMSCs on cancer cells are mediated through a secreted factor(s) which are enhanced by cancer cell-hMSC contact/communication. In addition to enhanced proliferation when in co-culture with hMSCs, MCF-7 cells were found to have increased migration potential in vitro. Inhibition of ER signalling by the pure anti-oestrogen ICI 182,780 decreased the effect of hMSCs on MCF-7 cell proliferation and migration supporting a role for ER signalling in the hMSC/MCF-7 cell interaction. Additionally, hMSCs have been shown to secrete a wide variety of growth factors and chemokines including stromal cell-derived factor-1 (SDF-1). This coupled with the knowledge that SDF-1 is an ER-mediated gene linked with hormone-independence and metastasis led to the investigation of the SDF-1/CXCR4 signalling axis in hMSC-MCF-7 cell interaction. Experiments revealed an increase in SDF-1 gene expression both in vivo and in vitro when MCF-7 cells were cultured with hMSCs. SDF-1 treatment of MCF-7 cells alone increased proliferation to just below that seen with hMSC co-culture. Additionally, blocking SDF-1 signalling using a CXCR4-specific inhibitor decreased hMSC induced proliferation and migration of MCF-7. However, the combined treatment of ICI and AMD3100 reduced MCF-7 cell proliferation and migration below control levels, indicating targeting both the ER and CXCR4 pathways is effective in decreasing the hMSCs induction of MCF-7 cell proliferation and migration.
Conclusions: The sum of these data reveals the relationship between tumour microenvironment and tumour growth and progression. Better understanding of the mechanisms involved in this tumour stroma cell interaction may provide novel targets for the development of treatment strategies for oestrogen receptor positive, hormone-independent, and endocrine-resistant breast carcinoma.
Figures

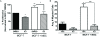
References
-
- Jordan VC. Long-term tamoxifen therapy to control or to prevent breast cancer: laboratory concept to clinical trials. Prog Clin Biol Res. 1988;262:105–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

