Exploring the molecular mechanisms underlying the potentiation of exogenous growth hormone on alcohol-induced fatty liver diseases in mice
- PMID: 21087523
- PMCID: PMC2994817
- DOI: 10.1186/1479-5876-8-120
Exploring the molecular mechanisms underlying the potentiation of exogenous growth hormone on alcohol-induced fatty liver diseases in mice
Abstract
Background: Growth hormone (GH) is an essential regulator of intrahepatic lipid metabolism by activating multiple complex hepatic signaling cascades. Here, we examined whether chronic exogenous GH administration (via gene therapy) could ameliorate liver steatosis in animal models of alcoholic fatty liver disease (AFLD) and explored the underlying molecular mechanisms.
Methods: Male C57BL/6J mice were fed either an alcohol or a control liquid diet with or without GH therapy for 6 weeks. Biochemical parameters, liver histology, oxidative stress markers, and serum high molecular weight (HMW) adiponectin were measured. Quantitative real-time PCR and western blotting were also conducted to determine the underlying molecular mechanism.
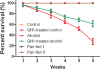
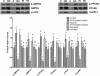
Results: Serum HMW adiponectin levels were significantly higher in the GH1-treated control group than in the control group (3.98 ± 0.71 μg/mL vs. 3.07 ± 0.55 μg/mL; P < 0.001). GH1 therapy reversed HMW adiponectin levels to the normal levels in the alcohol-fed group. Alcohol feeding significantly reduced hepatic adipoR2 mRNA expression compared with that in the control group (0.71 ± 0.17 vs. 1.03 ± 0.19; P < 0.001), which was reversed by GH therapy. GH1 therapy also significantly increased the relative mRNA (1.98 ± 0.15 vs. 0.98 ± 0.15) and protein levels of SIRT1 (2.18 ± 0.37 vs. 0.99 ± 0.17) in the alcohol-fed group compared with those in the control group (both, P < 0.001). The alcohol diet decreased the phosphorylated and total protein levels of hepatic AMP-activated kinase-α (AMPKα) (phosphorylated protein: 0.40 ± 0.14 vs. 1.00 ± 0.12; total protein: 0.32 ± 0.12 vs. 1.00 ± 0.14; both, P < 0.001) and peroxisome proliferator activated receptor-α (PPARα) (phosphorylated protein: 0.30 ± 0.09 vs. 1.00 ± 0.09; total protein: 0.27 ± 0.10 vs. 1.00 ± 0.13; both, P < 0.001), which were restored by GH1 therapy. GH therapy also decreased the severity of fatty liver in alcohol-fed mice.
Conclusions: GH therapy had positive effects on AFLD and may offer a promising approach to prevent or treat AFLD. These beneficial effects of GH on AFLD were achieved through the activation of the hepatic adiponectin-SIRT1-AMPK and PPARα-AMPK signaling systems.
Figures





Similar articles
-
Barley sprout extracts reduce hepatic lipid accumulation in ethanol-fed mice by activating hepatic AMP-activated protein kinase.Food Res Int. 2017 Nov;101:209-217. doi: 10.1016/j.foodres.2017.08.068. Epub 2017 Sep 12. Food Res Int. 2017. PMID: 28941686
-
Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats.Lipids Health Dis. 2010 Jul 26;9:78. doi: 10.1186/1476-511X-9-78. Lipids Health Dis. 2010. PMID: 20653983 Free PMC article.
-
Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice.Metabolism. 2013 Nov;62(11):1651-61. doi: 10.1016/j.metabol.2013.06.012. Epub 2013 Aug 5. Metabolism. 2013. PMID: 23928105
-
Adiponectin and alcoholic fatty liver disease.IUBMB Life. 2008 Dec;60(12):790-7. doi: 10.1002/iub.124. IUBMB Life. 2008. PMID: 18709650 Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Growth hormone reverses dyslipidemia in adult offspring after maternal undernutrition.Sci Rep. 2017 Jul 20;7(1):6038. doi: 10.1038/s41598-017-05045-1. Sci Rep. 2017. PMID: 28729704 Free PMC article.
-
Effect of nanoformulated copper/zinc superoxide dismutase on chronic ethanol-induced alterations in liver and adipose tissue.Alcohol. 2019 Sep;79:71-79. doi: 10.1016/j.alcohol.2018.12.005. Epub 2019 Jan 3. Alcohol. 2019. PMID: 30611703 Free PMC article.
-
Sirtuin 1 signaling and alcoholic fatty liver disease.Hepatobiliary Surg Nutr. 2015 Apr;4(2):88-100. doi: 10.3978/j.issn.2304-3881.2014.12.06. Hepatobiliary Surg Nutr. 2015. PMID: 26005675 Free PMC article. Review.
-
Schisandra chinensis prevents alcohol-induced fatty liver disease in rats.J Med Food. 2014 Jan;17(1):103-10. doi: 10.1089/jmf.2013.2849. J Med Food. 2014. PMID: 24456360 Free PMC article.
-
Relevance of the GH-VEGFB/VEGFA axis in liver grafts from brain-dead donors with alcohol-associated liver disease.Front Cell Dev Biol. 2025 Jan 7;12:1455258. doi: 10.3389/fcell.2024.1455258. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39839674 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

