The nature and combination of subunits used in epitope-based Schistosoma japonicum vaccine formulations affect their efficacy
- PMID: 21087526
- PMCID: PMC3136145
- DOI: 10.1186/1756-3305-3-109
The nature and combination of subunits used in epitope-based Schistosoma japonicum vaccine formulations affect their efficacy
Abstract
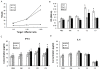
Background: Schistosomiasis remains a major public health problem in endemic countries and is caused by infections with any one of three primary schistosome species. Although there are no vaccines available to date, this strategy appears feasible since natural immunity develops in individuals suffering from repeated infection during a lifetime. Since vaccinations resulting in both Th1- and Th2-type responses have been shown to contribute to protective immunity, a vaccine formulation with the capacity for stimulating multiple arms of the immune response will likely be the most effective. Previously we developed partially protective, single Th- and B cell-epitope-based peptide-DNA dual vaccines (PDDV) (T3-PDDV and B3-PDDV, respectively) capable of eliciting immune responses against the Schistosoma japonicum 22.6 kDa tegument antigen (Sj22.6) and a 62 kDa fragment of myosin (Sj62), respectively.
Results: In this study, we developed PDDV cocktails containing multiple epitopes of S. japonicum from Sj22.6, Sj62 and Sj97 antigens by predicting cytotoxic, helper, and B-cell epitopes, and evaluated vaccine potential in vivo. Results showed that mice immunized with a single-epitope PDDV elicited either Tc, Th, or B cell responses, respectively, and mice immunized with either the T3- or B3- single-epitope PDDV formulation were partially protected against infection. However, mice immunized with a multicomponent (3 PDDV components) formulation elicited variable immune responses that were less immunoprotective than single-epitope PDDV formulations.
Conclusions: Our data show that combining these different antigens did not result in a more effective vaccine formulation when compared to each component administered individually, and further suggest that immune interference resulting from immunizations with antigenically distinct vaccine targets may be an important consideration in the development of multicomponent vaccine preparations.
Figures
References
LinkOut - more resources
Full Text Sources

