Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores
- PMID: 21087632
- PMCID: PMC3031774
- DOI: 10.1016/j.plipres.2010.10.004
Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores
Abstract
Lipolysis is the biochemical pathway responsible for the catabolism of triacylglycerol (TAG) stored in cellular lipid droplets. The hydrolytic cleavage of TAG generates non-esterified fatty acids, which are subsequently used as energy substrates, essential precursors for lipid and membrane synthesis, or mediators in cell signaling processes. Consistent with its central importance in lipid and energy homeostasis, lipolysis occurs in essentially all tissues and cell types, it is most abundant, however, in white and brown adipose tissue. Over the last 5years, important enzymes and regulatory protein factors involved in lipolysis have been identified. These include an essential TAG hydrolase named adipose triglyceride lipase (ATGL) [annotated as patatin-like phospholipase domain-containing protein A2], the ATGL activator comparative gene identification-58 [annotated as α/β hydrolase containing protein 5], and the ATGL inhibitor G0/G1 switch gene 2. Together with the established hormone-sensitive lipase [annotated as lipase E] and monoglyceride lipase, these proteins constitute the basic "lipolytic machinery". Additionally, a large number of hormonal signaling pathways and lipid droplet-associated protein factors regulate substrate access and the activity of the "lipolysome". This review summarizes the current knowledge concerning the enzymes and regulatory processes governing lipolysis of fat stores in adipose and non-adipose tissues. Special emphasis will be given to ATGL, its regulation, and physiological function.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
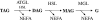
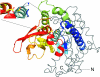
References
-
- Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. - PubMed
-
- Slawik M., Vidal-Puig A.J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5:144–164. - PubMed
-
- DeFronzo R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004:9–21. - PubMed
-
- Lelliott C., Vidal-Puig A.J. Lipotoxicity, an imbalance between lipogenesis de novo and fatty acid oxidation. Int J Obes Relat Metab Disord. 2004;28(Suppl. 4):S22–S28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

