Age-related gene response of human corneal endothelium to oxidative stress and DNA damage
- PMID: 21087955
- PMCID: PMC3101668
- DOI: 10.1167/iovs.10-6492
Age-related gene response of human corneal endothelium to oxidative stress and DNA damage
Abstract
Purpose: Nuclear oxidative DNA damage increases with age in human corneal endothelial cells (HCECs) and contributes to their decreased proliferative capacity. These studies investigated whether HCECs respond to this damage by upregulating their expression of oxidative stress and DNA damage-signaling genes in an age-dependent manner.
Methods: HCECs were dissected from the corneas of young (30 years and younger) and older (50 years and older) donors. Total RNA was isolated and reverse-transcribed. Oxidative stress and DNA damage-signaling gene expression were analyzed using commercial PCR-based microarrays. Western blot analyses were conducted on selected proteins to verify the microarray results. Nuclear DNA damage foci were detected in the endothelium of ex vivo corneas by immunostaining for H2AX-Ser139.
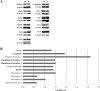
Results: Four of 84 genes showed a statistically significant age-related difference in the expression of oxidative stress-related genes; however, Western blot analysis demonstrated an age-related increase in only 2 (cytoglobin and GPX-1) of 11 proteins tested. No age-related differences were detected in the expression of DNA damage-signaling genes. Western blot analysis of seven DNA damage-related proteins verified this finding. Intense nuclear staining of DNA damage foci was observed in nuclei within the central endothelium of older donors. Central endothelium from young donors consistently showed a low level of positive staining.
Conclusions: HCECs respond to age-related increases in oxidative nuclear DNA damage by forming DNA damage repair foci; however, they do not vigorously defend against or repair this damage by upregulating the expression of multiple oxidative stress or DNA damage-signaling genes.
Figures



References
-
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–666 - PubMed
-
- Zhu CC, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45:1743–1751 - PubMed
-
- Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47:1387–1396 - PubMed
-
- Kikuchi M, Zhu C, Senoo T, Obara Y, Joyce NC. p27kip1 siRNA induces age-dependent proliferation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47:4803–4809 - PubMed
-
- Enomoto K, Mimura T, Harris DL, Joyce NC. Age-related differences in cyclin-dependent kinase inhibitor expression and retinoblastoma hyperphosphorylation in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47:4330–4340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

