Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival
- PMID: 21087957
- PMCID: PMC4183377
- DOI: 10.1167/iovs.10-6413
Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival
Abstract
Purpose: Human embryonic stem cell-derived RPE (hES-RPE) transplantation is a promising therapy for atrophic age-related macular degeneration (AMD); however, future therapeutic approaches may consider co-transplantation of hES-RPE with retinal progenitor cells (RPCs) as a replacement source for lost photoreceptors. The purpose of this study was to determine the effect of polarization of hES-RPE monolayers on their ability to promote survival of RPCs.
Methods: The hES-3 cell line was used for derivation of RPE. Polarization of hES-RPE was achieved by prolonged growth on permeable inserts. RPCs were isolated from 16- to 18-week-gestation human fetal eyes. ELISA was performed to measure pigment epithelium-derived factor (PEDF) levels from conditioned media.
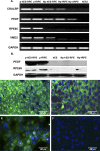
Results: Pigmented RPE-like cells appeared as early as 4 weeks in culture and were subcultured at 8 weeks. Differentiated hES-RPE had a normal chromosomal karyotype. Phenotypically polarized hES-RPE cells showed expression of RPE-specific genes. Polarized hES-RPE showed prominent expression of PEDF in apical cytoplasm and a marked increase in secretion of PEDF into the medium compared with nonpolarized culture. RPCs grown in the presence of supernatants from polarized hES-RPE showed enhanced survival, which was ablated by the presence of anti-PEDF antibody.
Conclusions: hES-3 cells can be differentiated into functionally polarized hES-RPE cells that exhibit characteristics similar to those of native RPE. On polarization, hES-RPE cells secrete high levels of PEDF that can support RPC survival. These experiments suggest that polarization of hES-RPE would be an important feature for promotion of RPC survival in future cell therapy for atrophic AMD.
Figures








Similar articles
-
Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration.Aging (Albany NY). 2009 Dec 27;2(1):28-42. doi: 10.18632/aging.100111. Aging (Albany NY). 2009. PMID: 20228934 Free PMC article.
-
Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution.Exp Eye Res. 2004 Feb;78(2):223-34. doi: 10.1016/j.exer.2003.10.013. Exp Eye Res. 2004. PMID: 14729355
-
Nicotine increases the VEGF/PEDF ratio in retinal pigment epithelium: a possible mechanism for CNV in passive smokers with AMD.Invest Ophthalmol Vis Sci. 2011 Jun 1;52(6):3842-53. doi: 10.1167/iovs.10-6254. Invest Ophthalmol Vis Sci. 2011. PMID: 21330654 Free PMC article.
-
Stem cell based therapies for age-related macular degeneration: The promises and the challenges.Prog Retin Eye Res. 2015 Sep;48:1-39. doi: 10.1016/j.preteyeres.2015.06.004. Epub 2015 Jun 23. Prog Retin Eye Res. 2015. PMID: 26113213 Review.
-
Delivery Systems of Retinoprotective Proteins in the Retina.Int J Mol Sci. 2021 May 19;22(10):5344. doi: 10.3390/ijms22105344. Int J Mol Sci. 2021. PMID: 34069505 Free PMC article. Review.
Cited by
-
Directional protein secretion by the retinal pigment epithelium: roles in retinal health and the development of age-related macular degeneration.J Cell Mol Med. 2013 Jul;17(7):833-43. doi: 10.1111/jcmm.12070. Epub 2013 May 11. J Cell Mol Med. 2013. PMID: 23663427 Free PMC article. Review.
-
Coculture techniques for modeling retinal development and disease, and enabling regenerative medicine.Stem Cells Transl Med. 2020 Dec;9(12):1531-1548. doi: 10.1002/sctm.20-0201. Epub 2020 Aug 7. Stem Cells Transl Med. 2020. PMID: 32767661 Free PMC article. Review.
-
Prominin-1 Is a Novel Regulator of Autophagy in the Human Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):2366-2387. doi: 10.1167/iovs.16-21162. Invest Ophthalmol Vis Sci. 2017. PMID: 28437526 Free PMC article.
-
Development of a new tissue injector for subretinal transplantation of human embryonic stem cell derived retinal pigmented epithelium.Int J Retina Vitreous. 2017 Oct 30;3:41. doi: 10.1186/s40942-017-0095-6. eCollection 2017. Int J Retina Vitreous. 2017. PMID: 29093829 Free PMC article.
-
Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization.J Biomed Biotechnol. 2012;2012:871272. doi: 10.1155/2012/871272. Epub 2012 May 22. J Biomed Biotechnol. 2012. PMID: 22685380 Free PMC article. Review.
References
-
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21 - PubMed
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 - PubMed
-
- Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina: relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30:1691–1699 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

