Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing
- PMID: 21087961
- PMCID: PMC3101689
- DOI: 10.1167/iovs.10-5866
Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing
Abstract
Purpose: Intracellular reactive oxygen species have been reported to associate with growth factor and integrin signalings in promoting cell adhesion in many cell types. This study is to explore if exogenous H(2)O(2) at low levels can be beneficial to cell adhesion, migration, and wound healing.
Methods: Primary rabbit corneal epithelial cells treated with 0-70 μM H(2)O(2) were tested for viability by MTT assay, adhesion by centrifugation assay, focal contacts of vinculin and F-actin by immunofluorescence, activated Src(pY416), EGF receptor (pY845), vinculin(pY1065), FAK(pY397), and FAK(pY576) by immunoblotting. Cell migration was examined with 0-50 μM H(2)O(2) using the scratch wound technique. Corneal wound healing of ex vivo pig model and in vivo mouse model was examined using H(2)O(2) with and without antioxidant N-acetylcysteine (NAC).
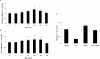
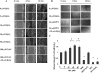
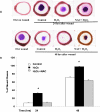
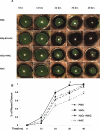
Results: Compared with the untreated control, H(2)O(2) at 10-50 μM stimulated cell viability and facilitated adhesion and migration with clear induction of vinculin-rich focal adhesions and F-actin-containing stress fibers by increasing activated Src, FAK(pY576), and vinculin(pY1065). H(2)O(2) also increased phosphorylation of EGFR(Y845) parallel to that of activated Src, but both were eliminated by NAC and PP1 (Src inhibitor). Finally, H(2)O(2) induced faster wound healing in cornea both in vitro and in vivo, but the healing was diminished by NAC.
Conclusions: These findings suggest that H(2)O(2) at low levels promotes cell adhesion, migration, and wound healing in cornea cells or tissue, and the interaction of H(2)O(2) with Src plays a major role.
Figures




Similar articles
-
Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5646-52. doi: 10.1167/iovs.08-2534. Epub 2009 Jun 3. Invest Ophthalmol Vis Sci. 2009. PMID: 19494198
-
Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing.Exp Eye Res. 2009 Dec;89(6):876-86. doi: 10.1016/j.exer.2009.07.012. Epub 2009 Jul 25. Exp Eye Res. 2009. PMID: 19635476
-
Reversal of second-hand cigarette smoke-induced impairment of corneal wound healing by thymosin beta4 combined with anti-inflammatory agents.Invest Ophthalmol Vis Sci. 2010 May;51(5):2424-35. doi: 10.1167/iovs.09-3692. Epub 2009 Dec 17. Invest Ophthalmol Vis Sci. 2010. PMID: 20019366
-
New insights in wound response and repair of epithelium.J Cell Physiol. 2013 May;228(5):925-9. doi: 10.1002/jcp.24268. J Cell Physiol. 2013. PMID: 23129239 Review.
-
Cell migration.Compr Physiol. 2012 Oct;2(4):2369-92. doi: 10.1002/cphy.c110012. Compr Physiol. 2012. PMID: 23720251 Free PMC article. Review.
Cited by
-
Enterococcus faecalis enhances cell proliferation through hydrogen peroxide-mediated epidermal growth factor receptor activation.Infect Immun. 2012 Oct;80(10):3545-58. doi: 10.1128/IAI.00479-12. Epub 2012 Jul 30. Infect Immun. 2012. PMID: 22851748 Free PMC article.
-
Think small: zebrafish as a model system of human pathology.J Biomed Biotechnol. 2012;2012:817341. doi: 10.1155/2012/817341. Epub 2012 Jun 3. J Biomed Biotechnol. 2012. PMID: 22701308 Free PMC article. Review.
-
Hydrogen Peroxide Generation of Copper/Ascorbate Formulations on Cotton: Effect on Antibacterial and Fibroblast Activity for Wound Healing Application.Molecules. 2018 Sep 19;23(9):2399. doi: 10.3390/molecules23092399. Molecules. 2018. PMID: 30235850 Free PMC article.
-
Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization.Am J Physiol Heart Circ Physiol. 2014 Oct 1;307(7):H945-57. doi: 10.1152/ajpheart.00918.2013. Epub 2014 Jul 25. Am J Physiol Heart Circ Physiol. 2014. PMID: 25063792 Free PMC article.
-
Tualang honey improves human corneal epithelial progenitor cell migration and cellular resistance to oxidative stress in vitro.PLoS One. 2014 May 6;9(5):e96800. doi: 10.1371/journal.pone.0096800. eCollection 2014. PLoS One. 2014. PMID: 24802273 Free PMC article.
References
-
- Cejkova J, Labsky J, Vacik J. Reactive oxygen species (ROS) generated by xanthine oxidase in the corneal epithelium and their potential participation in the damage of the corneal epithelium after prolonged use of contact lenses in rabbits. Acta Histochem. 1998;100:171–184 - PubMed
-
- Cejkova J, Stipek S, Crkovska J, Ardan T. Changes of superoxide dismutase, catalase and glutathione peroxidase in the corneal epithelium after UVB rays. Histochemical and biochemical study. Histol Histopathol. 2000;15:1043–1050 - PubMed
-
- Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902 - PubMed
-
- Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

